- Department of Neurosurgery Nara Medical University, Kashihara, Nara, Japan.
- Department of Radiology, Nara Medical University, Kashihara, Nara, Japan.
Correspondence Address:
Ichiro Nakagawa, Department of Neurosurgery, Nara Medical University, Kashihara, Nara, Japan.
DOI:10.25259/SNI_511_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takaaki Mitsui1, Ichiro Nakagawa1, Masashi Kotsugi1, HunSoo Park1, Shohei Yokoyama1, Kaoru Myouchin2, Hiroyuki Nakase1. Remarkable shrinkage of a thrombosed giant aneurysm by stent-assisted jam-packed coil embolization. 06-Jul-2021;12:328
How to cite this URL: Takaaki Mitsui1, Ichiro Nakagawa1, Masashi Kotsugi1, HunSoo Park1, Shohei Yokoyama1, Kaoru Myouchin2, Hiroyuki Nakase1. Remarkable shrinkage of a thrombosed giant aneurysm by stent-assisted jam-packed coil embolization. 06-Jul-2021;12:328. Available from: https://surgicalneurologyint.com/surgicalint-articles/10950/
Abstract
Background: Large and giant aneurysms are known to involve intra-aneurysmal thrombosis and present a poor prognosis because of compression of the surrounding brain tissue with enlargement of the aneurysm. These aneurysms are difficult to cure by endovascular treatment due to involvement of the vasa vasorum in their pathology. We report this technical note to describe stent-assisted jam-packed coil embolization for the treatment of a giant thrombosed aneurysm.
Case Description: A 62-year-old man presented with right homonymous hemianopsia, and magnetic resonance imaging (MRI) showed a giant thrombosed aneurysm with poor wall contrast enhancement, which indicates little involvement of the vasa vasorum, at the terminal part of the left internal carotid artery. To block blood flow into the aneurysmal dome, stent-assisted “jam-packed” coil embolization was performed. For this, a braided stent was shortened to enhance metal coverage ratio and tight aneurysmal coil packing was performed using a hydrogel coil. Our technique resulted in complete obliteration of the aneurysm, and MRI performed 1 year later showed remarkable shrinkage of the aneurysm dome.
Conclusion: Stent-assisted jam-packed coil embolization technique might be effective in shrinking the dome of giant thrombosed aneurysms with poor wall contrast enhancement.
Keywords: Jam-packed embolization, Thrombosed aneurysm, Vasa vasorum, Wall enhancement
INTRODUCTION
Giant intracranial aneurysms are rare, heterogeneous lesions with complex vascular anatomy.[
CASE REPORT
History and examination
A 62-year-old man with a history of hypertension and dyslipidemia became aware of visual field impairment and right upper limb weakness. 5 months later, he underwent a check-up at the hospital ophthalmology clinic, where he was diagnosed with right homonymous hemianopsia. Computed tomography (CT) showed an intracranial mass lesion, for which he was referred to the neurosurgical department of the same hospital. At that time, he had symptoms of the right homonymous hemianopsia and weakness of the right upper limb (manual motor test: 4/5).
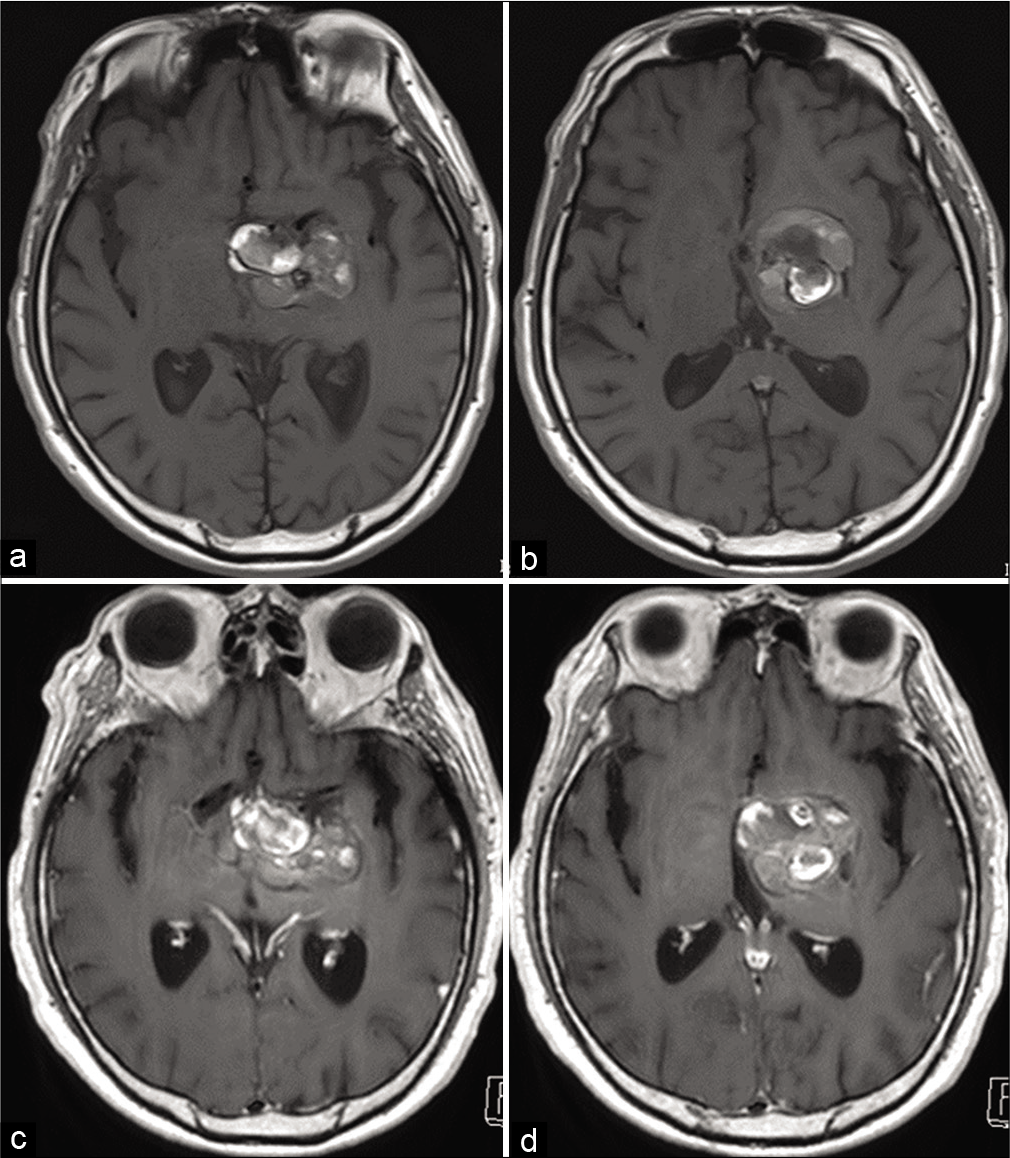
CT imaging revealed a multilobular heterogeneous density mass with a diameter of 38 mm × 33 mm × 23 mm, which compressed the ipsilateral basal ganglia. CT angiography showed unremarkable enhancement of the mass lesion. Head magnetic resonance (MR) T1-weighted imaging showed a heterogenous mixed intensity mass buried in the left basal ganglia, and compression of the optic nerve on the contralateral side. MR T2-weighted images showed findings suggestive of partial aneurysm wall hemorrhage, with no remarkable perifocal edema in the vicinity of the dome. Contrast-enhanced MR imaging (MRI) demonstrated poor contrast enhancement of the aneurysm wall which indicates little involvement of the vasa vasorum [
In such cases, the treatment strategy of direct surgical decompression of the aneurysm can be expected to immediately improve the symptoms caused by the mass effect. However, the procedure is associated with difficulty in securing the perforators in the vicinity of the aneurysm. Hence, we decided to perform stent-assisted “jam-packed” coil embolization with the purpose of interrupting the blood supply to the dome as soon as possible.
Endovascular procedure
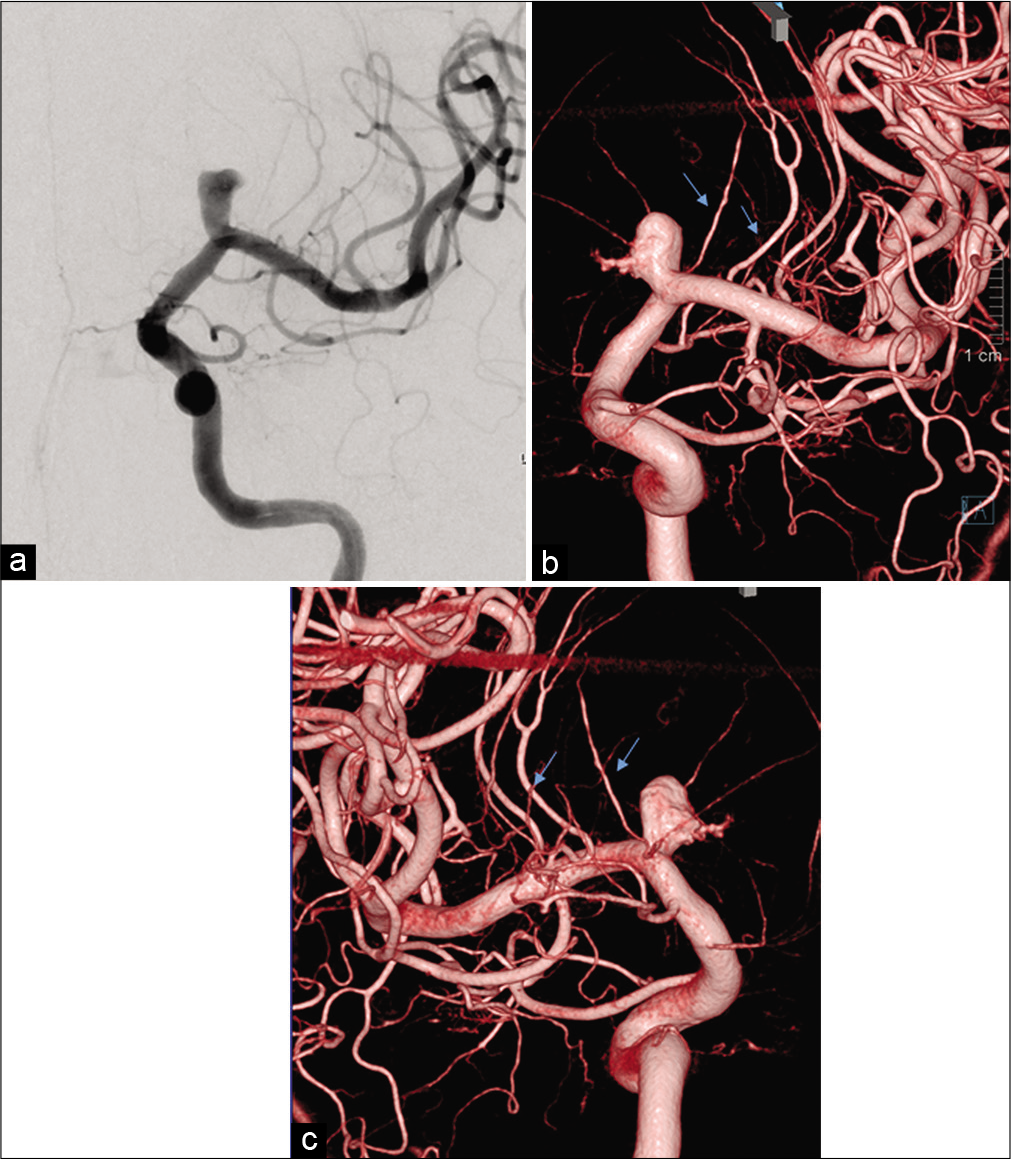
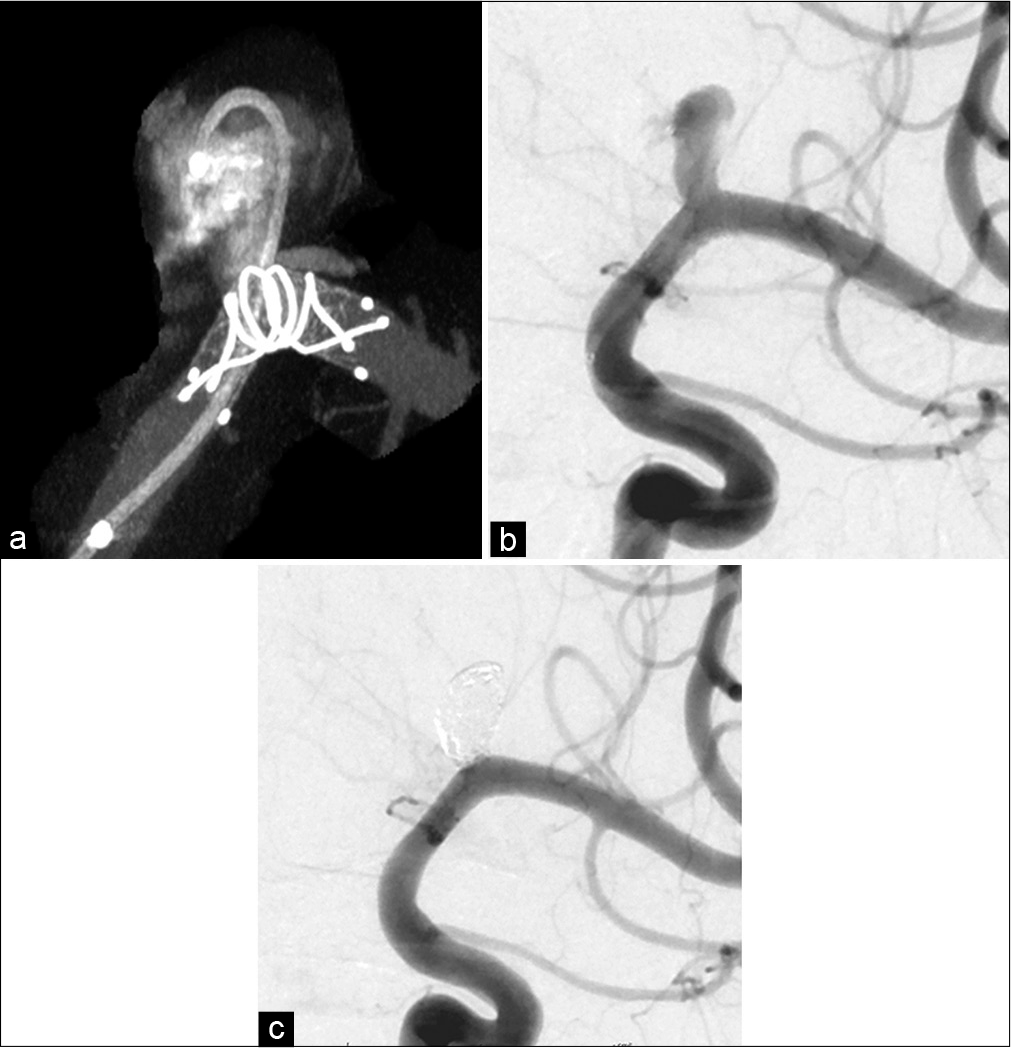
Aspirin (100 mg/day) and clopidogrel (75 mg/day) were administered a week before the procedure. An 8-Fr long sheath was placed in the right femoral artery under general anesthesia with motor evoked potential (MEP) monitoring. Heparinization was performed to maintain the activated clotting time at over 300 s. An 8-Fr guide catheter and 6-Fr distal access catheter were introduced into the left ICA, along with microcatheters for stenting (Headway 21, Terumo, Tokyo, Japan) and coil embolization (SL-10, Stryker, US). An LVIS stent (3.5 mm × 17 mm; Terumo, Tokyo, Japan) was deployed from the MCA proximal to the ICA with 25% shortening to enhance metal coverage ratio. using the push technique [
Outcome and follow-up
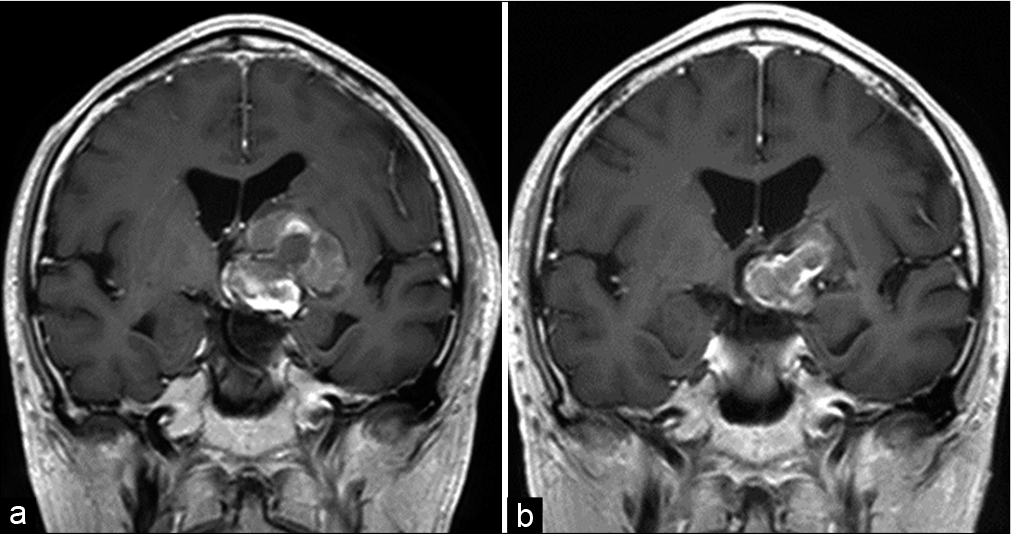
Postoperative diffusion-weighted imaging showed no new ischemic lesions and no new neurological symptoms. In addition, his preexisting symptoms were alleviated and he was subsequently discharged. 3 months after the treatment, his symptoms had completely recovered and DSA showed complete obliteration of the aneurysm and stent endothelialization. MRI performed 13 months after the treatment demonstrated remarkable shrinkage of the aneurysm [
DISCUSSION
Reportedly, 67% of enlarged thrombosed aneurysms present with serious neurological symptoms,[
The mechanisms of thrombosed giant cerebral aneurysm growth have been speculated in the previous literature. Ollikainen et al. pathologically reported the involvement of vasa vasorum,[
Flow diverter treatment for large and giant aneurysms has been previously reported, and a meta-analysis proved a high rate of aneurysm occlusion with the treatment.[
CONCLUSION
Stent-assisted jam-packed coil embolization technique might be effective for shrinking the dome of giant thrombosed aneurysms to immediately blocks blood flow into the aneurysm.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Becske T, Brinjikji W, Potts MB, Kallmes DF, Shapiro M, Moran CJ. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: Five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery. 2017. 80: 40-8
2. Becske T, Potts MB, Shapiro M, Kallmes DF, Brinjikji W, Saatci I. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg. 2017. 127: 81-8
3. Brinjikji W, Amar AP, Almandoz JE, Diaz O, Jabbour P, Hanel R. GEL THE NEC: A prospective registry evaluating the safety, ease of use, and efficacy of the HydroSoft coil as a finishing device. J Neurointerv Surg. 2018. 10: 83-7
4. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke. 2013. 44: 442-7
5. Dengler J, Maldaner N, Glasker S, Endres M, Wagner M, Malzahn U. Outcome of surgical or endovascular treatment of giant intracranial aneurysms, with emphasis on age, aneurysm location, and unruptured aneuryms-a systematic review and meta-analysis. Cerebrovasc Dis. 2016. 41: 187-98
6. Dengler J, Rufenacht D, Meyer B, Rohde V, Endres M, Lenga P. Giant intracranial aneurysms: Natural history and 1-year case fatality after endovascular or surgical treatment. J Neurosurg. 2019. 134: 49-57
7. Drake CG. Giant intracranial aneurysms: Experience with surgical treatment in 174 patients. Clin Neurosurg. 1979. 26: 12-95
8. Drake CG. Progress in cerebrovascular disease. Management of cerebral aneurysm. Stroke. 1981. 12: 273-83
9. Ferns SP, Sprengers ME, van Rooij WJ, Rinkel GJ, van Rijn JC, Bipat S. Coiling of intracranial aneurysms: A systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009. 40: e523-9
10. Ferns SP, van Rooij WJ, Sluzewski M, van den Berg R, Majoie CB. Partially thrombosed intracranial aneurysms presenting with mass effect: Long-term clinical and imaging follow-up after endovascular treatment. AJNR Am J Neuroradiol. 2010. 31: 1197-205
11. Foreman PM, Salem MM, Griessenauer CJ, Dmytriw AA, Parra-Farinas C, Nicholson P. Flow diversion for treatment of partially thrombosed aneurysms: A multicenter cohort. World Neurosurg. 2020. 135: e164-73
12. Guresir E, Wispel C, Borger V, Hadjiathanasiou A, Vatter H, Schuss P. Treatment of partially thrombosed intracranial aneurysms: Single-center series and systematic review. World Neurosurg. 2018. 118: e834-41
13. Iihara K, Murao K, Yamada N, Takahashi JC, Nakajima N, Satow T. Growth potential and response to multimodality treatment of partially thrombosed large or giant aneurysms in the posterior circulation. Neurosurgery. 2008. 63: 832-42
14. Krings T, Alvarez H, Reinacher P, Ozanne A, Baccin CE, Gandolfo C. Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol. 2007. 13: 117-26
15. Lawton MT, Quinones-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms: Classification scheme and management strategies in 68 patients. Neurosurgery. 2005. 56: 441-54
16. Lubicz B, Leclerc X, Gauvrit JY, Lejeune JP, Pruvo JP. Giant vertebrobasilar aneurysms: Endovascular treatment and long-term follow-up. Neurosurgery. 2004. 55: 316-23
17. Matsushige T, Chen B, Ringelstein A, Umutlu L, Forsting M, Quick HH. Giant intracranial aneurysms at 7T MRI. AJNR Am J Neuroradiol. 2016. 37: 636-41
18. Nurminen V, Lehecka M, Chakrabarty A, Kivisaari R, Lehto H, Niemela M. Anatomy and morphology of giant aneurysms-angiographic study of 125 consecutive cases. Acta Neurochir (Wien). 2014. 156: 1-10
19. Ollikainen E, Tulamo R, Frosen J, Lehti S, Honkanen P, Hernesniemi J. Mast cells, neovascularization, and microhemorrhages are associated with saccular intracranial artery aneurysm wall remodeling. J Neuropathol Exp Neurol. 2014. 73: 855-64
20. Poncyljusz W, Bilinski P, Safranow K, Baron J, Zbroszczyk M, Jaworski M. The LVIS/LVIS Jr. stents in the treatment of wide-neck intracranial aneurysms: Multicentre registry. J Neurointerv Surg. 2015. 7: 524-9
21. Sato T, Matsushige T, Chen B, Gembruch O, Dammann P, Jabbarli R. Wall contrast enhancement of thrombosed intracranial aneurysms at 7T MRI. AJNR Am J Neuroradiol. 2019. 40: 1106-11