- Department of Neurosurgery, Universidade Federal de São Paulo,
- Department of Neurosurgery, Faculdade de Medicina de São Jose do Rio Preto, Sao Jose do Rio Preto,
- Department of Neurosurgery, Centro Hospitalar Tondela-Viseu, EPE, Viseu, Portugal.
- Department of Neurosurgery, Universidade de São Paulo, São Paulo, Brazil,
Correspondence Address:
Marcos Devanir Silva da Costa
Department of Neurosurgery, Universidade de São Paulo, São Paulo, Brazil,
DOI:10.25259/SNI_133_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Feres Chaddad-Neto1, Marcos Devanir Silva da Costa1, Bruno Santos1, Ricardo Lourenco Caramanti2, Bruno Lourenco Costa3, Hugo Leonardo Doria-Netto1, Eberval Gadelha Figueiredo4. Reproducibility of a new classification of the anterior clinoid process of the sphenoid bone. 12-Sep-2020;11:281
How to cite this URL: Feres Chaddad-Neto1, Marcos Devanir Silva da Costa1, Bruno Santos1, Ricardo Lourenco Caramanti2, Bruno Lourenco Costa3, Hugo Leonardo Doria-Netto1, Eberval Gadelha Figueiredo4. Reproducibility of a new classification of the anterior clinoid process of the sphenoid bone. 12-Sep-2020;11:281. Available from: https://surgicalneurologyint.com/surgicalint-articles/reproducibility-of-a-new-classification-of-the-anterior-clinoid-process-of-the-sphenoid-bone/
Abstract
Background: Pneumatization of the anterior clinoid process (ACP) affects paraclinoid region surgery, this anatomical variation occurs in 6.6–27.7% of individuals, making its preoperative recognition essential given the need for correction based on the anatomy of the pneumatized process. This study was conducted to evaluate the reproducibility of an optic strut-based ACP pneumatization classification by presenting radiological examinations to a group of surgeons.
Methods: Thirty cranial computer tomography (CT) scans performed from 2013 to 2014 were selected for analysis by neurosurgery residents and neurosurgeons. The evaluators received Google Forms with questionnaires on each scan, DICOM files to be manipulated in the Horos software for multiplanar reconstruction, and a collection of slides demonstrating the steps for classifying each type of ACP pneumatization. Interobserver agreement was calculated by the Fleiss kappa test.
Results: Thirty CT scans were analyzed by 37 evaluators, of whom 20 were neurosurgery residents and 17 were neurosurgeons. The overall reproducibility of the ACP pneumatization classification showed a Fleiss kappa index of 0.49 (95% confidence interval: 0.49–0.50). The interobserver agreement indices for the residents and neurosurgeons were 0.52 (0.51–0.53) and 0.49 (0.48–0.50), respectively, and the difference was statistically significant (P
Conclusion: The optic strut-based classification of ACP pneumatization showed acceptable concordance. Minor differences were observed in the agreement between the residents and neurosurgeons. These differences could be explained by the residents’ presumably higher familiarity with multiplanar reconstruction software.
Keywords: Anterior clinoid process, Sphenoid bone, Cavernous sinus, Skull base, Optic strut
INTRODUCTION
The anterior clinoid process (ACP) protrudes posteriorly from the lesser sphenoid wing of the sphenoid bone, composing the anterior portion of the cavernous sinus’ roof. The base of the ACP has three attachment points with the adjacent sphenoid bone: The lateral attachment is the medial border of the lesser sphenoid wing and, laterally, the anterior root of the lesser sphenoid wing extends from the base of the ACP to the sphenoid body, forming the roof of the optic canal, also called the planum sphenoidale. The third point is a minor sphenoid bone, called the optic strut, that extends below the optic nerve, reaching the body of the sphenoid and forming the floor of the optic canal and roof of the superior orbital fissure.[
The optic pillar is a small bony bridge that extends from the inferomedial surface of the ACP base to the sphenoid body, immediately ahead of the carotid sulcus. From its junction with the ACP, the optic strut slopes gently downward as it approaches the body of the sphenoid.
Within the context of the anatomy of the sphenoid bone, the ACP has strategic importance for the surgical approach since its removal is a critical step in the treatment of paraclinoid lesions, which are closely related to the ACP, required to gain a partial medial view of the minor wing of the sphenoid bone and the cavernous sinus roof. Examples of conditions requiring ACP removal include aneurysms of the ophthalmic segment of the internal carotid artery, meningiomas of the cavernous sinus, and the medial third of the lesser sphenoid wing and also giant pituitary adenomas.[
The microsurgical procedure of anterior clinoidectomy is a key to the treatment of pathologies that arise from the paraclinoid region. However, this procedure involves the risks of visual disturbances, oculomotor nerve palsy/paralysis, bleeding from the opening of the cavernous sinus, lesion of the internal carotid artery as well as of the ophthalmic artery, opening of the paranasal sinuses, and cerebrospinal fluid fistula potentially leading to meningitis and death. For paraclinoid aneurysm surgery, the reported rates of morbidity, mortality, and cerebrospinal fluid fistula with or without meningitis are 5.8– 18%, 0.6–45.4%, and 2.2–14%, respectively. Because anterior clinoidectomy is imperative for paraclinoid aneurysms, these rates are likely to be similar to those in patients with paraclinoid diseases undergoing surgery.[
Pneumatization of the ACP is an anatomical variation that occurs in 6.6–27.7% of individuals, making its preoperative recognition essential given the need for correction based on the anatomy of the pneumatized process.[
MATERIALS AND METHODS
Patients were selected from project (morphometric analysis of the ACP of the sphenoid bone), approved by the Institutional Research Ethics Committee. These patients received skull base computed tomography (CT) scans (internal and mastoid ears), because of suspected internal/mastoid ear disease, at the Department of Radiology and Diagnostic Imaging from January 1, 2013, to September 6, 2014. Patients with a history of paranasal sinus disease who had undergone transsphenoidal surgery, as well as neurosurgical procedures involving the skull base, were excluded from the study.
Images of skull base tomography (internal and mastoid) were obtained at the Department of Radiology and Diagnostic Imaging, using the Brilliance computed tomography (CT) 64 system (Philips) with the following technical specifications: collimation 20 × 0.625, pitch 0.348, matrix 512, field of view 200 mm, 140 kpv, 278–600 mA, and cut thickness 0.67 mm. Pneumatization was characterized by the presence of structures with the same air density.
Each evaluator received a Google Forms email link (
Three senior neurosurgeons, authors of the original ACP pneumatic classification paper,[
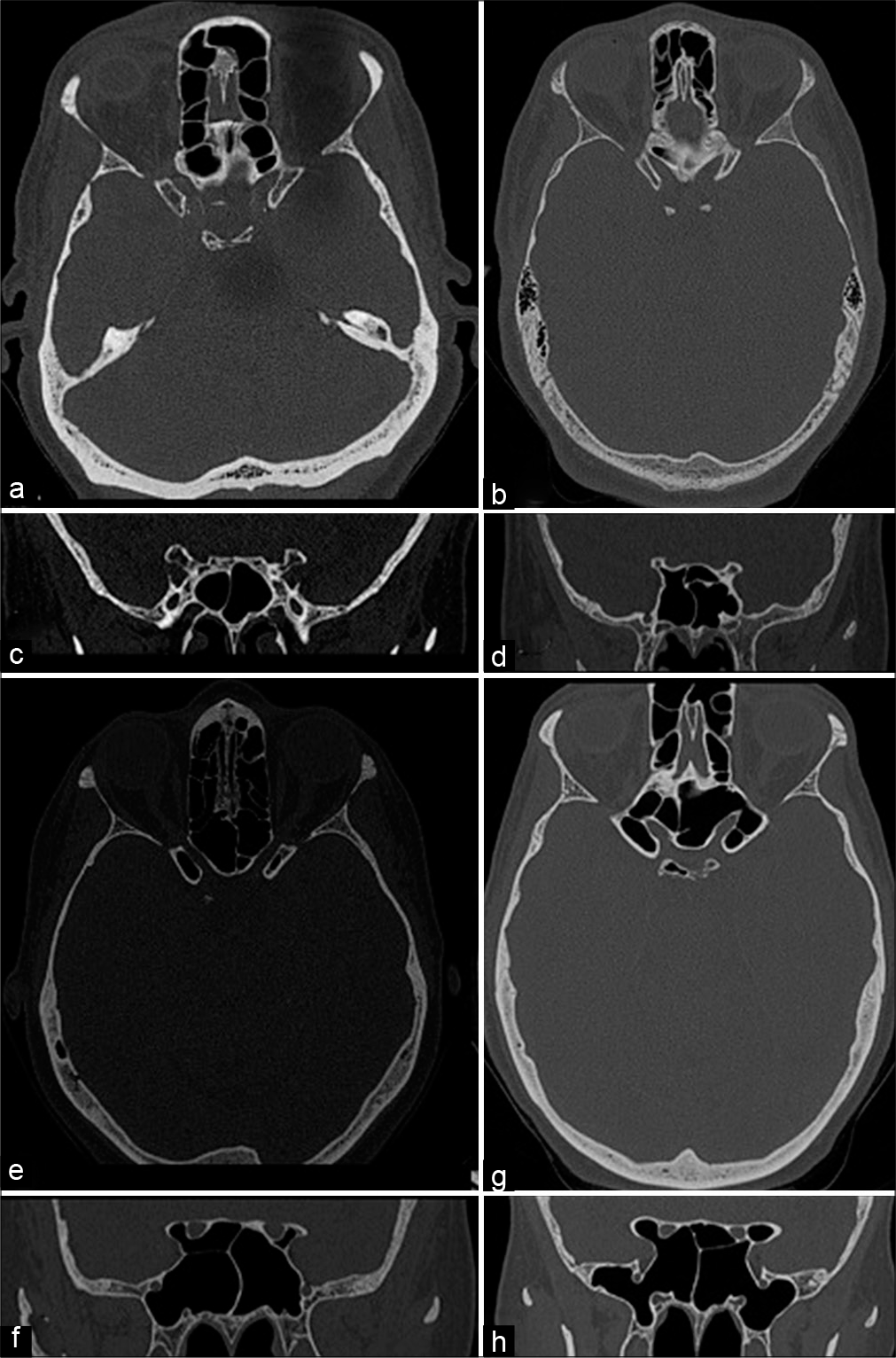
Figure 1:
This is a panel of skull-based computed tomography containing all the examples of each type of anterior clinoid. (a and b) The axial and coronal views of a Type 0, absence of pneumatization. (c and d)The axial and coronal views of a Type 1, pneumatization of the optic strut without anterior clinoid pneumatization. (e and f) The axial and coronal views of Type 2A (left side) and 2B (right side), this subtype presents a pneumatized anterior clinoid and optic strut, the difference is the volume of pneumatization in the anterior clinoid, the Type 2A has less than 50% and the Type 2b has more than 50% of aeration. (g and h)The axial and coronal views of Type 3, pneumatization of the anterior clinoid, optic strut, and planum sphenoidale.
Descriptive statistics are presented as numbers or frequencies as appropriate.
RESULTS
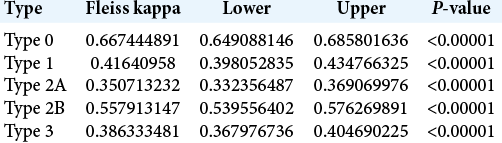
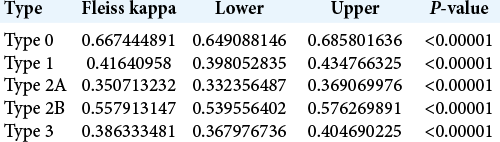
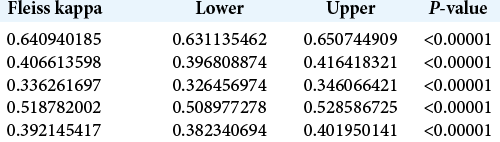
The 30 skull-based CT scans were analyzed by a total of 37 evaluators, of which 20 (54%) were neurosurgery residents and 17 (46%) were neurosurgeons. In the overall analysis of the classification, the Fleiss kappa index of interobserver agreement was 0.49 (95% CI: 0.49–0.50). However, when the neurosurgeons and residents were analyzed separately, the respective Fleiss kappa indices of interobserver agreement were 0.49 (95% CI: 0.48–0.50) and 0.52 (95% CI: 0.51–0.53), and the differences between the general interobserver agreement index and those among the neurosurgery residents and neurosurgeons were statistically significant with P < 0.00001 [
The overall analysis of all 37 assessors in relation to each subtype of ACP pneumatization classification revealed that Type 0 (complete lack of pneumatization) showed the substantial agreement of 0.66 (95% CI: 0.64–0.68) and Type 2A demonstrated the fair agreement of 0.35 (95% CI: 0.33–0.36). The other subtypes showed fair to moderate concordance, summarized in [
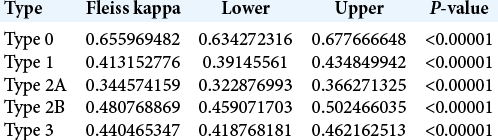
The same trends were found when analyzing the subtypes by evaluator group. Among the neurosurgeons [
DISCUSSION
In an interobserver analysis considering all evaluators, the reproducibility of the optic strut-based ACP pneumatization classification was moderate, with a Fleiss kappa value of 0.49 (95% CI: 0.49–0.50), which represents acceptable interobserver reproducibility. As stated in the original report of this classification, it is intended as a preoperative guideline for surgery involving pathologies of the paraclinoid region, which entails opening the anterior portion of the cavernous sinus roof and anterior clinoidectomy.[
At least three other clinoid pneumatization classifications have been proposed;[
Among the different subtypes of the classification, 2A showed the worst interobserver agreement indices, followed by 3, 1, 2B, and 0. Type 0 predictably had the best reproducibility since it represents the absence of changes or pneumatization. In contrast to 2B, subtype 2A showed only fair reproducibility. Type 3 was not highly prevalent in the sample and requires better knowledge of the DICOM viewer program; it was moderately difficult to classify.
The practical aspect of this anterior clinoid classification is the possibility of preoperative planning for the correction of possible openings or communication of the sphenoid and ethmoid sinuses in surgeries of pathologies involving the paraclinoid region, which requires an anterior clinoidectomy. In this sense, type 0 indicates that there will be no need for any intraoperative repair, unless the neurosurgeon intentionally wishes to open one of these paranasal sinuses. In Types 1, 2 A, and 2 B, despite the difference in the degree of pneumatization, the pneumatization route is the same, the optic strut, and therefore, the repair technique must be focused on closing the optic strut. In type 3, the correction should require a technique that includes closing the opening of the planum sphenoidale with communication to the ethmoid sinus, and the optic strut with communication to the sphenoid sinus, [
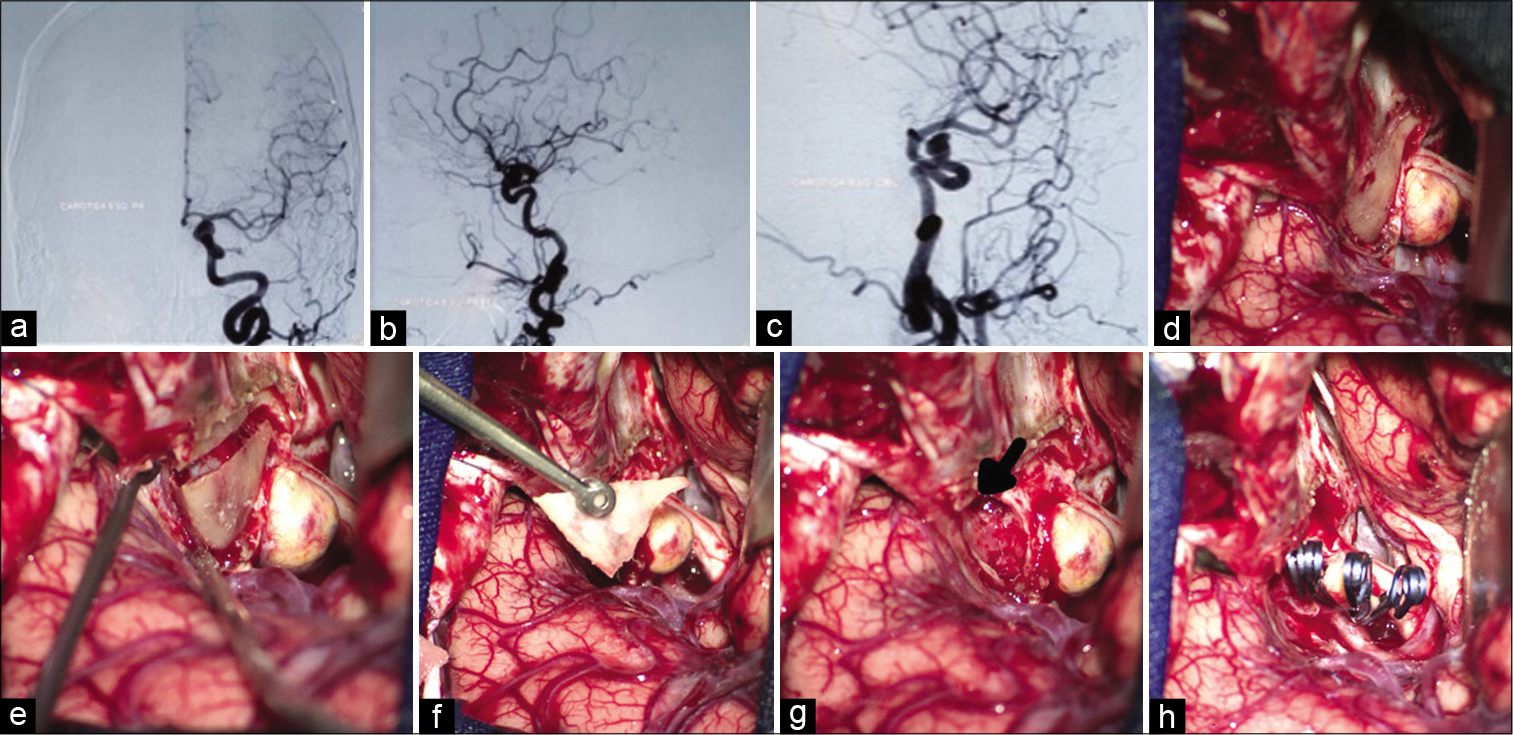
Figure 2:
This panel of images illustrates a clinical case of a 56-year-old female that presented with visual loss of the left eye, the angiogram (a-c) showed a paraclinoid aneurysm. The image (d) shows the intraoperative exposure of the anterior clinoid process. The image (e) reveals the disconnection of the anterior clinoid process from its bone attachments. The image (f) shows the “en bloc” removal of the anterior clinoid process. The image (g) reveals the communication of the sphenoid sinus through the optic strut promoted by the anterior clinoid removal. Figure (h) shows the final aspect of the aneurysm clipping.
Further study is needed to analyze the effect of combining subtypes 2A and 2B into a single category on the reproducibility of the optic pillar-based pneumatic ACP classification. Because classification was based on a single contact with the CT images in the present study, future research should also address the possibility of improving reproducibility through multiple rounds of image evaluation by the same observer.
CONCLUSION
We found moderate reproducibility of the optic strut-based classification of ACP pneumatization. This classification represents a simple and sufficiently reproducible method of preoperative analysis of patient imaging data to be applied in clinical practice for planning surgery in the paraclinoid region.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abuzayed B, Tanriover N, Biceroglu H, Yuksel O, Tanriover O, Albayram S. Pneumatization degree of the anterior clinoid process: A new classification. Neurosurg Rev. 2010. 33: 367-73
2. Chi JH, Sughrue M, Kunwar S, Lawton MT. The yo-yo technique to prevent cerebrospinal fluid rhinorrhea after anterior clinoidectomy for proximal internal carotid artery aneurysms. Neurosurgery. 2006. 59: ONS101-7
3. Colli BO, Carlotti CG, Assirati JA, Abud DG, Amato MC, Dezena RA. Results of microsurgical treatment of paraclinoid carotid aneurysms. Neurosurg Rev. 2013. 36: 99-114
4. da Costa MD, Santos BF, Paz DA, Rodrigues TP, Abdala N, Centeno R. Anatomical variations of the anterior clinoid process: A study of 597 skull base computerized tomography scans. Oper Neurosurg (Hagerstown). 2016. 12: 289-97
5. Day AL. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990. 72: 677-91
6. De Jesus O, Sekhar LN, Riedel CJ. Clinoid and paraclinoid aneurysms: Surgical anatomy, operative techniques, and outcome. Surg Neurol. 1999. 51: 477-87
7. Dolenc VV. A combined epi-and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985. 62: 667-72
8. Drake CG, Vanderlinden RG, Amacher AL. Carotid-ophthalmic aneurysms. J Neurosurg. 1968. 29: 24-31
9. Fleiss JL.editors. Statistical Methods for Rates and Proportions. New York: John Wiley & Sons; 1981. p.
10. Giannotta SL. Ophthalmic segment aneurysm surgery. Neurosurgery. 2002. 50: 558-62
11. Javalkar V, Banerjee AD, Nanda A. Paraclinoid carotid aneurysms. J Clin Neurosci. 2011. 18: 13-22
12. Kulwin C, Tubbs RS, Cohen-Gadol AA. Anterior clinoidectomy: Description of an alternative hybrid method and a review of the current techniques with an emphasis on complication avoidance. Surg Neurol Int. 2011. 2: 140
13. Lai LT, Morgan MK. Outcomes for unruptured ophthalmic segment aneurysm surgery. J Clin Neurosci. 2013. 20: 1127-33
14. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33: 159-74
15. Lehmberg J, Krieg SM, Meyer B. Anterior clinoidectomy. Acta Neurochir (Wien). 2014. 156: 415-9
16. Mikami T, Minamida Y, Koyanagi I, Baba T, Houkin K. Anatomical variations in pneumatization of the anterior clinoid process. J Neurosurg. 2007. 106: 170-4
17. Nanda A, Javalkar V. Microneurosurgical management of ophthalmic segment of the internal carotid artery aneurysms: Single-surgeon operative experience from Louisiana State University, Shreveport. Neurosurgery. 2011. 68: 355-70
18. Ota N, Tanikawa R, Miyazaki T, Miyata S, Oda J, Noda K. Surgical microanatomy of the anterior clinoid process for paraclinoid aneurysm surgery and efficient modification of extradural anterior clinoidectomy. World Neurosurg. 2015. 83: 635-43
19. Raco A, Frati A, Santoro A, Vangelista T, Salvati M, Delfini R. Long-term surgical results with aneurysms involving the ophthalmic segment of the carotid artery. J Neurosurg. 2008. 108: 1200-10
20. Rhoton AL Jr. The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery. 2002. 51: S375-410
21. Rhoton AL Jr. The sellar region. Neurosurgery. 2002. 51: S335-74
22. Sharma BS, Kasliwal MK, Suri A, Chandra PS, Gupta A, Mehta VS. Outcome following surgery for ophthalmic segment aneurysms. J Clin Neurosci. 2010. 17: 38-42
23. Silveira RL, Gusmao S, Pinheiro N, Andrade GC. Paraclinoid aneurysms: Surgical technique and results in 51 patients. Arq Neuropsiquiatr. 2004. 62: 322-9
24. Xu BN, Sun ZH, Romani R, Jiang JL, Wu C, Zhou DB. Microsurgical management of large and giant paraclinoid aneurysms. World Neurosurg. 2010. 73: 137-46