- Departments of Neurosurgery, Tokyo Metropolitan Police Hospital, 4-22-1 Nakano, Nakano-ku, Tokyo, Japan.
- Departments of Diagnostic Pathology, Tokyo Metropolitan Police Hospital, 4-22-1 Nakano, Nakano-ku, Tokyo, Japan.
Correspondence Address:
Keisuke Nagata
Departments of Neurosurgery, Tokyo Metropolitan Police Hospital, 4-22-1 Nakano, Nakano-ku, Tokyo, Japan.
DOI:10.25259/SNI_259_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Keisuke Nagata, Satoshi Kiyofuji, Munehiro Yokoyama, Shigeo Sora. Resection of a lateral supratentorial endodermal cyst complicated by postoperative seizures: A case report. 19-Jul-2019;10:141
How to cite this URL: Keisuke Nagata, Satoshi Kiyofuji, Munehiro Yokoyama, Shigeo Sora. Resection of a lateral supratentorial endodermal cyst complicated by postoperative seizures: A case report. 19-Jul-2019;10:141. Available from: http://surgicalneurologyint.com/surgicalint-articles/9517/
Abstract
Background: Endodermal cysts are uncommon cystic lesions usually located at the ventral aspects of the spine. A lateral supratentorial location of such cysts is extremely rare. A unique case of a lateral supratentorial endodermal cyst that required surgical intervention due to uncal herniation, complicated with postoperative seizures, is presented.
Case Description: A 48-year-old man presented with transient motor aphasia and diplopia. Magnetic resonance imaging showed a cystic lesion occupying the left frontal and temporal convexity with midline shift and uncal herniation. Cyst resection was performed, and cyst contents with mucous-like components were aspirated. Histopathological examination showed an endodermal cyst. The patient showed no neurological deficits immediately after surgery but developed tonic-clonic seizures 9 h after surgery. Sedation and intubation were required to control the seizures. After administering multiple antiepileptic drugs, he was extubated on the 5th day after surgery. He was discharged home in a month with mild impairment in dexterity of his right hand.
Conclusions: Surgical intervention for endodermal cysts can be complicated by postoperative seizures caused by chemical irritation of brain cortex due to spillage of cyst contents. It is important to irrigate the cyst wall very well intraoperatively and pay attention not to spill the cyst fluid to unaffected locations. Preoperative administration of antiepileptic drugs should also be considered if endodermal cysts, not simple arachnoid cysts, are suspected preoperatively.
Keywords: Endodermal cyst, Neurenteric cyst, Postoperative seizure, Status epilepticus
INTRODUCTION
Endodermal cysts are rare, benign, cystic lesions that commonly arise at the ventral aspects of the spine. There have been reports of intracranial lesions, but they tend to be localized in the midline in the infratentorial compartment, and lateral supratentorial endodermal cysts are even rarer. Total or subtotal cyst resection leads to satisfactory postsurgical outcomes, but periprocedural complications such as seizures sometimes occur. A unique case of a surgically treated lateral supratentorial endodermal cyst followed by postoperative status epilepticus is presented.
CASE PRESENTATION
Patient history
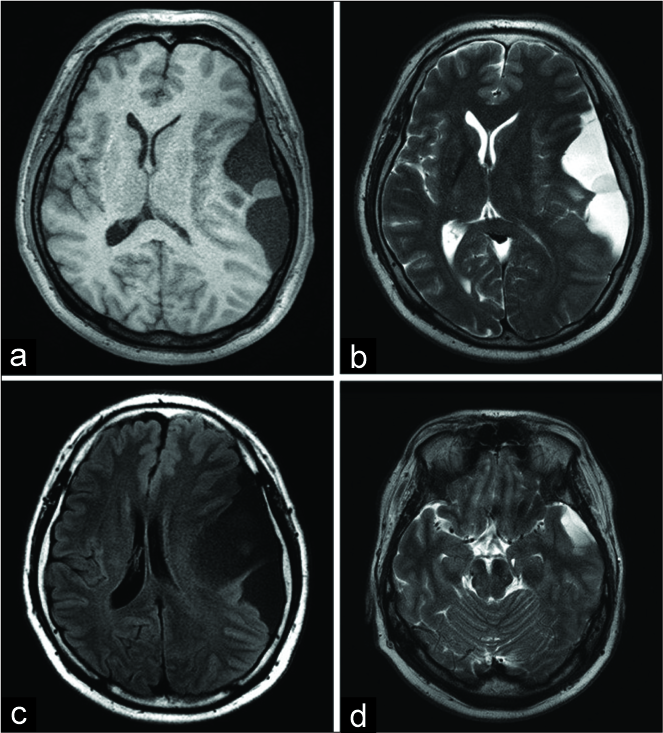
The patient was a 48-year-old man with no significant medical history who presented with transient motor aphasia. On neurological examination, diplopia was noted on the right lateral gaze, which suggested partial left oculomotor nerve palsy, with no dysfunction of pupillary reflexes. Head magnetic resonance imaging (MRI) showed a large supratentorial cystic lesion that occupied the left temporal and frontal convexity, with a 10-mm midline shift and uncal herniation. The cyst contents showed low intensity on T1-weighted images [
Figure 1:
Preoperative magnetic resonance imaging showing T1- weighted (a), T2-weighted (b), and fluid-attenuated inversion recovery (c) images. The cyst content demonstrates slightly higher intensity than regular cerebrospinal fluid on fluid-attenuated inversion recovery images, containing a mucous-like nodule with isointensity on T1WI and mild high intensity on T2WI. Uncal herniation is observed at the level of the mesencephalon (d).
It was concluded that the mass effect from the cyst caused uncal herniation [
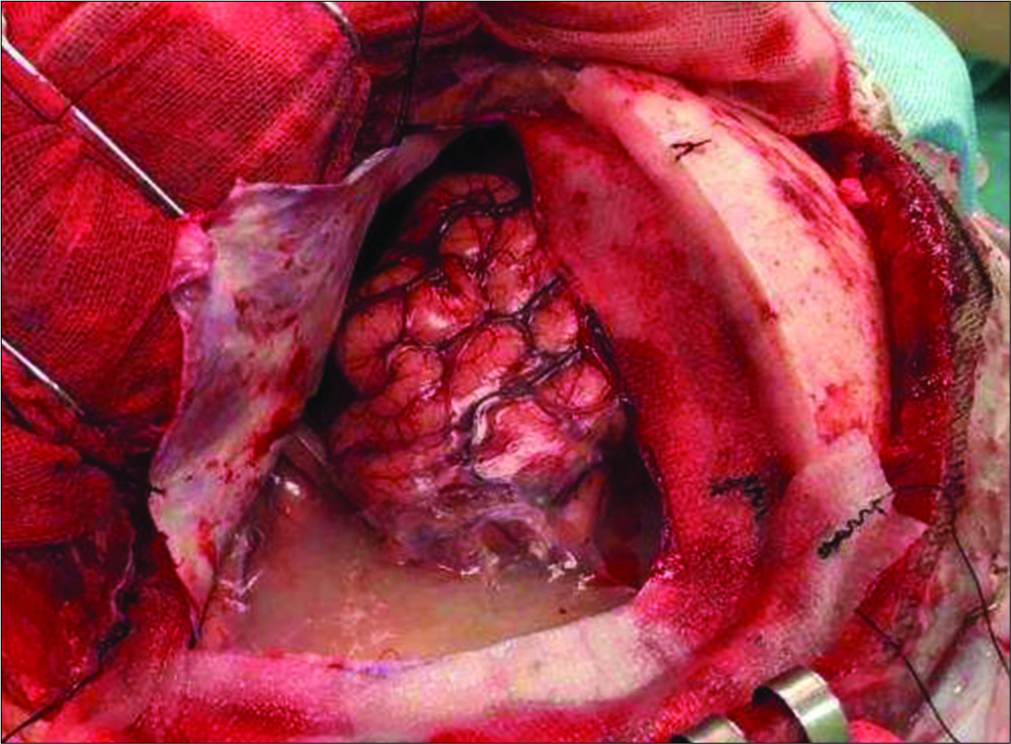
Surgery
Under general anesthesia, a left frontotemporal craniotomy was performed. As the dura mater was opened, a large extra- axial cyst was seen, covered with a thin whitish membrane and containing white turbid fluid [
Postoperative course
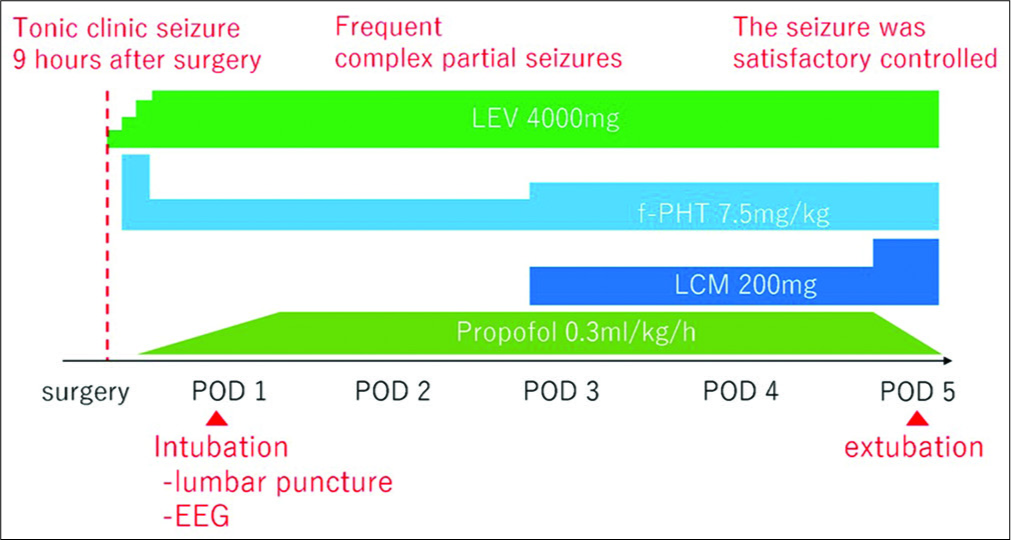
The patient did not demonstrate any neurological deficits immediately after surgery. However, he experienced a tonic- clonic seizure 9 h after surgery. Diazepam and levetiracetam (LEV) were immediately administered, but frequent complex partial seizures still occurred. Fosphenytoin (f-PHT) was also administered, and he was intubated and sedated with propofol. Lumbar puncture was performed on postoperative day 1, and the obtained CSF was whitish and opaque, with the initial pressure as high as 330 mmH2O. The cell count was 11/uL, glucose was 68 mg/dL, and protein was 46.6 mg/dL. The electroencephalogram showed theta bursts localized in bilateral frontal lobes. Status epilepticus caused by chemical meningitis was diagnosed. The seizure was finally satisfactorily controlled with LEV 4000 mg, f-PHT 7.5 mg/kg, lacosamide 200 mg, and propofol 0.3 mg/kg/h, with minimal simple partial seizures affecting the right hand. Propofol was stopped on the 5th day, and the patient was extubated. Postoperative course shown above is organized as a timeline [
Figure 3:
Postoperative course is organized as a timeline. The patient experienced a tonic-clonic seizure 9 h after surgery. Lumbar puncture and electroencephalogram were performed on postoperative day 1. The seizure was finally controlled with levetiracetam 4000 mg, Fosphenytoin 7.5 mg/kg, Lacosamide 200 mg, and propofol 0.3 mg/kg/h.
Pathological findings
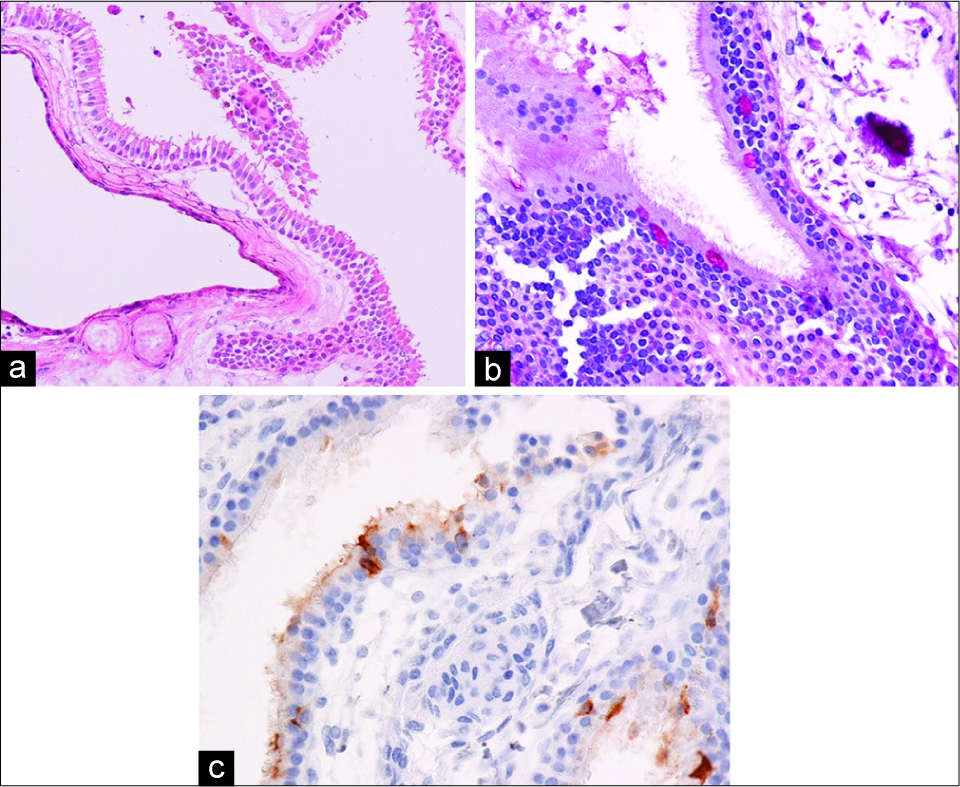
On histopathological examination, the cyst wall contained pseudostratified ciliated columnar epithelium with a basement membrane on hematoxylin and eosin staining [
Figure 5:
Histopathological features of the cyst wall. Hematoxylin and eosin staining shows pseudostratified ciliated columnar epithelium with a basement membrane (a). The presence of goblet cells is shown by periodic acid-Schiff staining (b). Carcinoembryonic antigen is also positive in the cytoplasm in a few epithelial cells, suggesting carcinoembryonic antigen secretory capacity (c).
DISCUSSION
Endodermal cysts often occur at the ventral aspects of the spine, especially at the lower cervical and upper thoracic levels. Intracranial lesions are rare, and they have usually been reported in the infratentorial compartments such as the posterior fossa or craniocervical junction, especially in the midline. A lateral supratentorial location is extremely rare for endodermal cysts.
The pathogenesis of endodermal cysts is not yet fully understood. There have been three major hypotheses regarding the origins of endodermal cysts. The predominantly supported hypothesis is that they originate from the neurenteric canal,[
Endodermal cysts are often misdiagnosed as arachnoid cysts due to the CSF-like intensity of their contents on MRI. In some cases, the cyst fluid shows a slightly higher intensity than CSF on T1-weighted images, reflecting the protein-rich nature of the cyst contents. Some previous studies showed a nodule with high intensity on T2WI, surrounded by fluid, as in the present case.[
There is no established treatment strategy for lateral supratentorial endodermal cysts due to their rarity. Surgical resection is thought to lead to satisfactory outcomes with a low recurrence rate. Góes et al. collected 45 cases of endodermal cysts that were supratentorial in origin.[
In the present case, intractable seizures continued for 3 days after surgery, probably due to chemical irritation to the brain. To prevent these complications, we suggest that it is important to irrigate the cyst wall very well and pay attention to not spill the whitish cloudy fluid onto unaffected areas. Preoperative administration of antiepileptic drugs can also be considered.
CONCLUSIONS
Surgical intervention for endodermal cysts can be complicated by postoperative seizures caused by chemical irritation of brain cortex due to spillage of cyst contents. Careful irrigation of the cyst wall and preoperative administration of antiepileptic drugs should be considered if endodermal cysts are suspected preoperatively.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank the staff at FORTE Science Communications www.forte-science.co.jp for providing language help.
References
1. Akimoto J, Nakajima N, Saida A, Miki T, Haraoka J. Symptomatic suprasellar endodermal cyst, possibly originating from the seessel’s pouch, containing fluid with a high carcinoembryonic antigen level. Brain Tumor Pathol. 2013. 30: 128-33
2. Chen CT, Lai HY, Jung SM, Lee CY, Wu CT, Lee ST. Neurenteric cyst or neuroendodermal cyst? Immunohistochemical study and pathogenesis. World Neurosurg. 2016. 96: 85-90
3. Cheng JS, Cusick JF, Ho KC, Ulmer JL. Lateral supratentorial endodermal cyst: Case report and review of literature. Neurosurgery. 2002. 51: 493-9
4. Christov C, Chrétien F, Brugieres P, Djindjian M. Giant supratentorial enterogenous cyst: Report of a case, literature review, and discussion of pathogenesis. Neurosurgery. 2004. 54: 759-63
5. Góes P, Vaz-Guimaraes F, Suriano IC, Araújo S, Zymberg ST. Supratentorial neurenteric cyst: Analysis of 45 cases in the literature. Interdiscip Neurosurg. 2018. 11: 57-64
6. Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965. 122: 467-81
7. Graziani N, Dufour H, Figarella-Branger D, Donnet A, Bouillot P, Grisoli F. Do the suprasellar neurenteric cyst, the rathke cleft cyst and the colloid cyst constitute a same entity?. Acta Neurochir (Wien). 1995. 133: 174-80
8. Kachur E, Ang LC, Megyesi JF. Intraparenchymal supratentorial neurenteric cyst. Can J Neurol Sci. 2004. 31: 412-6
9. Krishnamurthy G, Kumar VR, Rajeswaran R, Rao S. Supratentorial enterogenous cyst: A report of two cases and review of literature. Neurol India. 2010. 58: 774-7