- Department of Neurosurgery, Riverside University Health System, Moreno Valley, California, United States,
- Department of Neurology, Kaiser Permanente Sourthern California Physician Medical Group, Los Angeles, California, United States,
- Department of Neurosurgery, Kaiser Permanente Los Angeles Medical Center, Los Angeles, California, United States.
Correspondence Address:
Paras Savla
Department of Neurosurgery, Riverside University Health System, Moreno Valley, California, United States.
DOI:10.25259/SNI_723_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samir Kashyap1, Rita Ceponiene2, Paras Savla1, Jacob Bernstein1, Hammad Ghanchi1, Ajay Ananda3. Resolution of tardive tremor after bilateral subthalamic nucleus deep brain stimulation placement. 16-Dec-2020;11:444
How to cite this URL: Samir Kashyap1, Rita Ceponiene2, Paras Savla1, Jacob Bernstein1, Hammad Ghanchi1, Ajay Ananda3. Resolution of tardive tremor after bilateral subthalamic nucleus deep brain stimulation placement. 16-Dec-2020;11:444. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10457
Abstract
Background: Tardive tremor (TT) is an underrecognized manifestation of tardive syndrome (TS). In our experience, TT is a rather common manifestation of TS, especially in a setting of treatment with aripiprazole, and is a frequent cause of referrals for the evaluation of idiopathic Parkinson disease. There are reports of successful treatment of tardive orofacial dyskinesia and dystonia with deep brain stimulation (DBS) using globus pallidus interna (GPi) as the primary target, but the literature on subthalamic nucleus (STN) DBS for tardive dyskinesia (TD) is lacking. To the best of our knowledge, there are no reports on DBS treatment of TT.
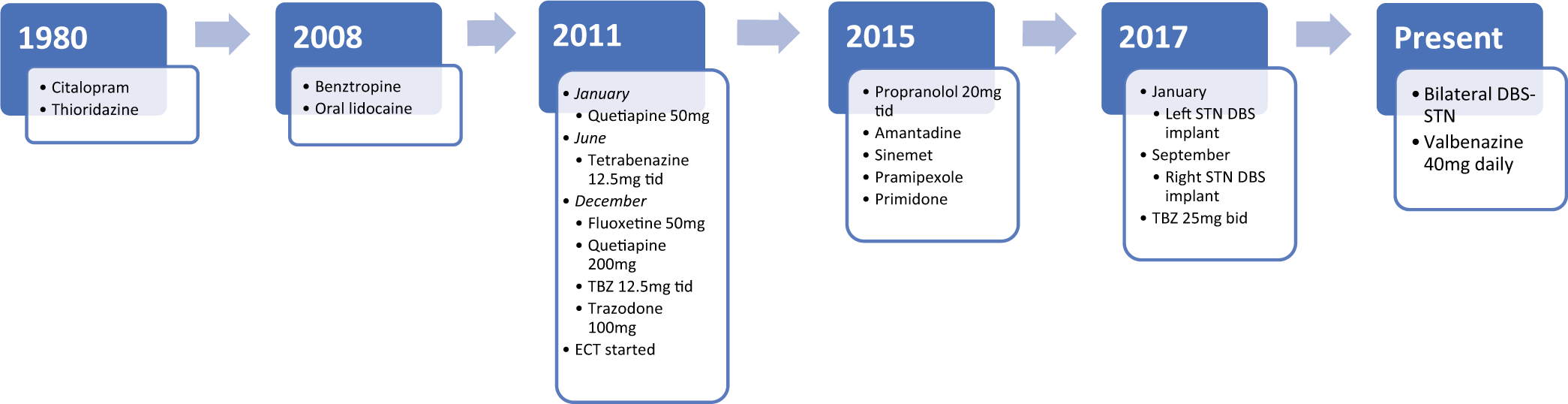
Case Description: A 75-year-old right-handed female with the medical history of generalized anxiety disorder and major depressive disorder had been treated with thioridazine and citalopram from 1980 till 2010. Around 2008, she developed orolingual dyskinesia. She was started on tetrabenazine in June 2011. She continued to have tremors and developed Parkinsonian gait, both of which worsened overtime. She underwent DBS placement in the left STN in January 2017 with near-complete resolution of her tremors. She underwent right STN implantation in September 2017 with similar improvement in symptoms.
Conclusion: While DBS-GPi is the preferred treatment in treating oral TD and dystonia, DBS-STN could be considered a safe and effective target in patients with predominating TT and/or tardive Parkinsonism. This patient saw a marked improvement in her symptoms after implantation of DBS electrodes, without significant relapse or recurrence in the years following implantation.
Keywords: Deep brain stimulation, Subthalamic nucleus, Tardive dyskinesia, Tardive tremor
INTRODUCTION
Tardive dyskinesia (TD) is a complex iatrogenic movement disorder that can manifest as orolingual, truncal, or respiratory dyskinesia as well as dystonia, tremor, chorea, myoclonus, akathisia, or Parkinsonism.[
Tardive tremor (TT) is an underrecognized manifestation of tardive syndrome (TS). It was first described by Stacy and Jankovic and in a few following reports.[
There are reports of successful treatment of tardive orofacial dyskinesia and dystonia with deep brain stimulation (DBS) using globus pallidus interna (GPi) as the primary target, but the literature on subthalamic nucleus (STN) DBS for TD is lacking.[
CLINICAL PRESENTATION
A 75-year-old right-handed female with the medical history of generalized anxiety disorder and major depressive disorder with a history of SI has been treated with thioridazine and citalopram from 1980 till 2010. Around 2008 at the age of 67, she developed orolingual dyskinesia: “difficulty with her tongue thrusting out of her mouth, causing the tongue to be sore and lips to be chapped, and annoying her.” Her treatment and symptom timeline are summarized in [
Video 1
A possibility of therapeutic DBS surgery was discussed and the patient was eager to get the treatment. Her dopamine transporter (DAT) scan showed normal DAT in basal ganglia. One possibility for this patient was to target the GPi with the hopes of alleviating her oTD and discontinuing treatment with TBZ. However, due to the two trials of TBZ discontinuation not alleviating the tremors, this was considered too risky. Therefore, a decision was made to target the STN with the goal of alleviating her main complaint which was resting leg and hand tremor, as well as bradykinesia and rigidity.
In January 2017, she underwent left STN implantation with Medtronic DBS system, with initial near-complete resolution of tremors. In September 2017, she underwent right STN implantation with similar improvement, however with the side effect of marked hypophonia and mild cognitive impact [
Video 2
DISCUSSION
The incidence of tardive symptoms has been reported as high as one in three patients on typical antipsychotics.[
DIP is another condition that is associated with antipsychotic use, affecting up to 4–40% of patients. In addition, TBZ, the most effective medication for oTD, has also been reported to induce Parkinsonism in approximately 30% of patients.[
Typically, DBS is considered if medical treatment fails or if medication adverse effects are intolerable to the patient. The common targets for electrode placement, as in PD, are the GPi or the STN; however, the vast majority of research has been conducted in tardive dystonia and has not been well studied in oral manifestations of TD. In a sham stimulation-controlled trial, 25 patients with oral TD and tardive dystonia were randomized into a sham (no stimulation for 3 months) and active treatment group of pallidal DBS. At 6 months, all patients showed 41.5% improvement in symptoms with active treatment.[
Prior studies have shown that STN-DBS is beneficial in treating not only resting but also postural and kinetic tremors.[
CONCLUSION
Tardive movement disorders are complex conditions that require highly personalized management. In addition to well-known manifestations with tardive oral dyskinesia and tardive dystonia, they may include the less acknowledged TTs and tardive Parkinsonism. While DBS-GPi is the preferred treatment in treating oral TD and dystonia, DBS-STN could be considered a safe and effective target in patients with predominating TT and/or tardive Parkinsonism.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
References
1. Ba F, Martin WR. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat Disord. 2015. 21: 87-94
2. Bhidayasiri R, Boonyawairoj S. Spectrum of tardive syndromes: Clinical recognition and management. Postgrad Med J. 2011. 87: 132-41
3. Bhidayasiri R, Jitkritsadakul O, Friedman JH, Fahn S. Updating the recommendations for treatment of tardive syndromes: A systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018. 389: 67-75
4. Blanchet P, Kivenko V. Drug-induced parkinsonism: Diagnosis and management. JPRLS. 2016. 6: 83-91
5. Diamond A, Shahed J, Jankovic J. The effects of subthalamic nucleus deep brain stimulation on parkinsonian tremor. J Neurol Sci. 2007. 260: 199-203
6. Eltahawy HA, Feinstein A, Khan F, Saint-Cyr J, Lang AE, Lozano AM. Bilateral globus pallidus internus deep brain stimulation in tardive dyskinesia: A case report. Mov Disord. 2004. 19: 969-72
7. Gruber D, Südmeyer M, Deuschl G, Falk D, Krauss JK, Mueller J. Neurostimulation in tardive dystonia/ dyskinesia: A delayed start, sham stimulation-controlled randomized trial. Brain Stimul. 2018. 11: 1368-77
8. Kanodia S, Guha S. Tardive dyskinesia responsive to deep brain stimulation. Aust N Z J Psychiatry. 2018. 52: 717
9. Kefalopoulou Z, Paschali A, Markaki E, Vassilakos P, Ellul J, Constantoyannis C. A double-blind study on a patient with tardive dyskinesia treated with pallidal deep brain stimulation. Acta Neurol Scand. 2009. 119: 269-73
10. Kertesz DP, Swartz MV, Tadger S, Plopski I, Barak Y. Tetrabenazine for tardive tremor in elderly adults: A prospective follow-up study. Clin Neuropharmacol. 2015. 38: 23-5
11. Macerollo A, Deuschl G. Deep brain stimulation for tardive syndromes: Systematic review and meta-analysis. J Neurol Sci. 2018. 389: 55-60
12. Mentzel CL, Tenback DE, Tijssen MA, Visser-Vandewalle VE, van Harten PN. Efficacy and safety of deep brain stimulation in patients with medication-induced tardive dyskinesia and/or dystonia: A systematic review. J Clin Psychiatry. 2012. 73: 1434-8
13. Miguel R, Mendonça MD, Barbosa R, Ladeira F, Lampreia T, Vale J. Tetrabenazine in treatment of hyperkinetic movement disorders: An observational study. Ther Adv Neurol Disord. 2017. 10: 81-90
14. Paleacu D, Giladi N, Moore O, Stern A, Honigman S, Badarny S. Tetrabenazine treatment in movement disorders. Clin Neuropharmacol. 2004. 27: 230-3
15. Parihar R, Alterman R, Papavassiliou E, Tarsy D, Shih LC. Comparison of VIM and STN DBS for parkinsonian resting and postural/action tremor. Tremor Other Hyperkinet Mov (N Y). 2015. 5: 321
16. Pouclet-Courtemanche H, Rouaud T, Thobois S, Nguyen JM, Brefel-Courbon C, Chereau I. Long-term efficacy and tolerability of bilateral pallidal stimulation to treat tardive dyskinesia. Neurology. 2016. 86: 651-9
17. Shin HW, Chung SJ. Drug-induced parkinsonism. J Clin Neurol. 2012. 8: 15-21
18. Shuaib UA, Rajput AH, Robinson CA, Rajput A. Neuroleptic-induced parkinsonism: Clinicopathological study. Mov Disord. 2016. 31: 360-5
19. Spindler MA, Galifianakis NB, Wilkinson JR, Duda JE. Globus pallidus interna deep brain stimulation for tardive dyskinesia: Case report and review of the literature. Parkinsonism Relat Disord. 2013. 19: 141-7
20. Stacy M, Jankovic J. Tardive tremor. Mov Disord. 1992. 7: 53-7
21. Sun B, Chen S, Zhan S, Le W, Krahl SE. Subthalamic nucleus stimulation for primary dystonia and tardive dystonia. Acta Neurochir Suppl. 2007. 97: 207-14
22. Tarsy D, Indorf G. Tardive tremor due to metoclopramide. Mov Disord. 2002. 17: 620-1
23. Ure RJ, Dhanju S, Lang AE, Fasano A. Unusual tremor syndromes: Know in order to recognise. J Neurol Neurosurg Psychiatry. 2016. 87: 1191-203
24. Waln O, Jankovic J. An update on tardive dyskinesia: From phenomenology to treatment. Tremor Other Hyperkinet Mov (N Y). 2013. 3: tre-03-161-4138-1
25. Welter ML, Grabli D, Vidailhet M. Deep brain stimulation for hyperkinetics disorders: Dystonia, tardive dyskinesia, and tics. Curr Opin Neurol. 2010. 23: 420-5
26. Wirshing WC, Freidenberg DL, Cummings JL, Bartzokis G. Effects of anticholinergic agents on patients with tardive dyskinesia and concomitant drug-induced parkinsonism. J Clin Psychopharmacol. 1989. 9: 407-11
27. Zhang WF, Tan YL, Zhang XY, Chan RC, Wu HR, Zhou DF. Extract of Ginkgo biloba treatment for tardive dyskinesia in schizophrenia: A randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011. 72: 615-21
28. Zheng W, Xiang YQ, Ng CH, Ungvari GS, Chiu HF, Xiang YT. Extract of Ginkgo biloba for tardive dyskinesia: Meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2016. 49: 107-11
29. Zutshi D, Cloud LJ, Factor SA. Tardive syndromes are rarely reversible after discontinuing dopamine receptor blocking agents: Experience from a university-based movement disorder clinic. Tremor Other Hyperkinet Mov (N Y). 2014. 4: 266