- Department of Neurosurgery, Global Neurosciences Institute, Philadelphia, Pennsylvania,
- Department of Cell Biology and Neuroscience, Rowan University School of Osteopathic Medicine, Stratford, New Jersey,
- Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, Wisconsin, United States.
Correspondence Address:
Hirad S. Hedayat, Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, Wisconsin, United States.
DOI:10.25259/SNI_135_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Christina R. Maxwell1, Neal Joshi2, Christina N. Feller3, Michael McAree2, Hirad S. Hedayat3. Reversal of cognitive, behavioral, and language impairments after the left frontal arachnoid cyst fenestration in a pediatric patient. 27-Jul-2021;12:371
How to cite this URL: Christina R. Maxwell1, Neal Joshi2, Christina N. Feller3, Michael McAree2, Hirad S. Hedayat3. Reversal of cognitive, behavioral, and language impairments after the left frontal arachnoid cyst fenestration in a pediatric patient. 27-Jul-2021;12:371. Available from: https://surgicalneurologyint.com/surgicalint-articles/11002/
Abstract
Background: Arachnoid cysts (ACs) are cerebrospinal fluid-containing cysts located between the surface of the brain or spinal cord and arachnoid layer of the leptomeninges. ACs have been known to cause cognitive, language, and behavioral deficits and currently there is no standard treatment paradigm. Surgical indications include papilledema, increasing growth with mass effect causing neurological deficit, or rapid head growth, however, cognitive symptoms related to mass effect may not always be considered.
Case Description: We present a 3-year-old male with an AC of the left anterior fossa causing frontal lobe compression with resultant behavioral, language, and cognitive deficits.
Conclusion: Surgical intervention for AC decompression may be indicated when there are cognitive, behavioral, or language delays related to the mass effect and location of the AC. Neuropsychiatric testing or more advanced imaging studies may further support surgical treatment. After craniotomy for fenestration of the left frontal AC, there was drastic improvement in cognitive, language, and behavioral symptoms in our pediatric patient.
Keywords: Arachnoid cyst, Case report, Craniotomy, Fenestration, Neurosurgery, Pediatrics
INTRODUCTION
Arachnoid cysts (ACs) are cerebrospinal fluid containing cysts located between the surface of the brain or spinal cord and arachnoid layer of the meninges. The majority of ACs are congenital malformations of the leptomeninges, two-thirds of which arise in the temporal fossa. A minority of ACs arise from secondary causes such as trauma, meningitis, intracranial hemorrhage, or after neurological surgery.[
We present the first case of significant improvement of cognitive, behavioral, and language delays in a pediatric patient after surgical decompression of an AC of the anterior fossa compressing the frontal lobe. In addition, we discuss the role for surgical intervention in pediatric patients with AC in the presence of cognitive and language dysfunction when more common surgical indications such as growth on serial imaging studies, focal neurological deficits from mass effect, or papilledema are absent.
CASE PRESENTATION
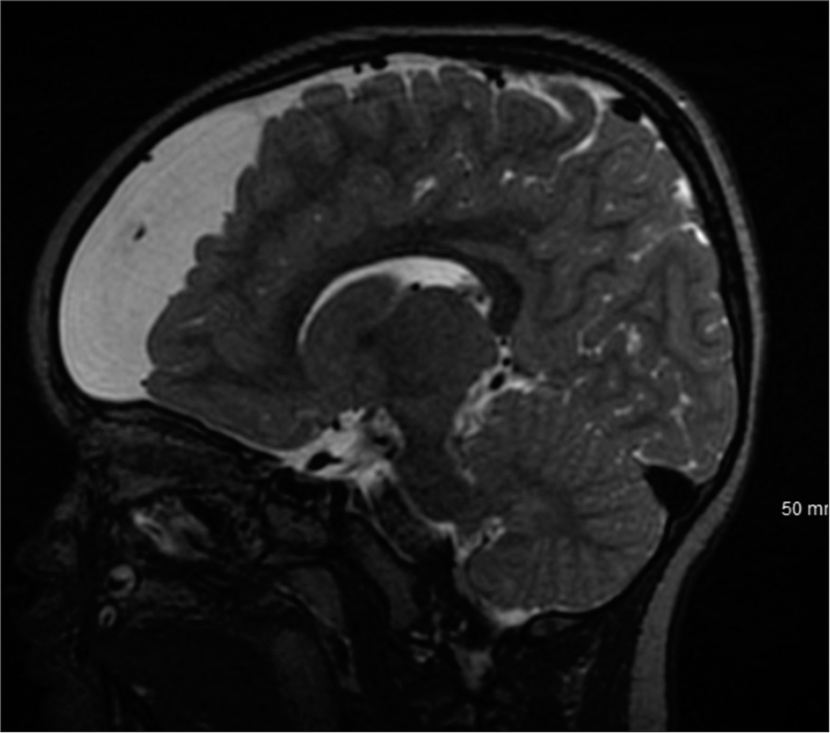
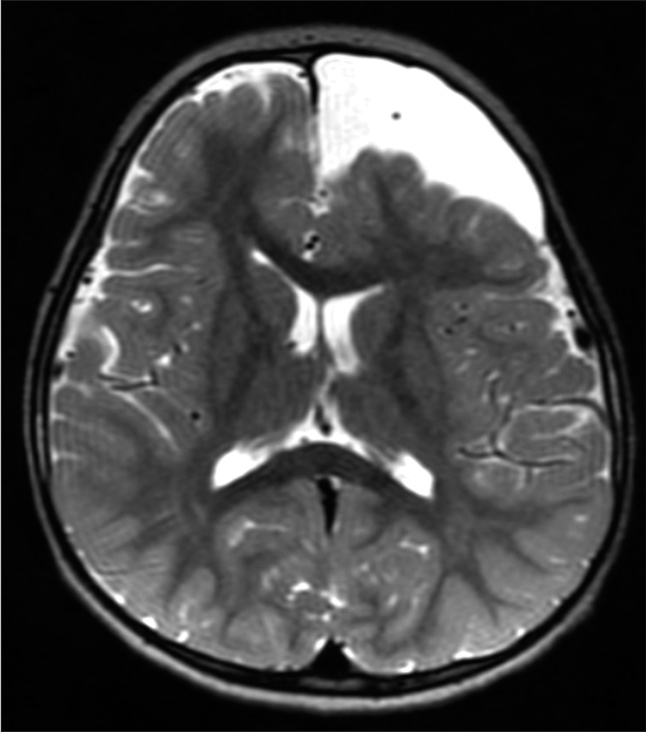
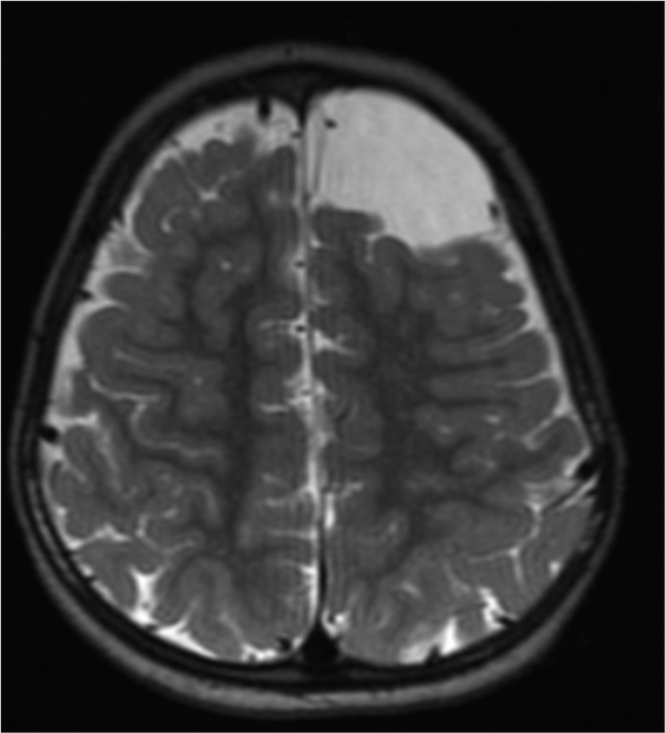
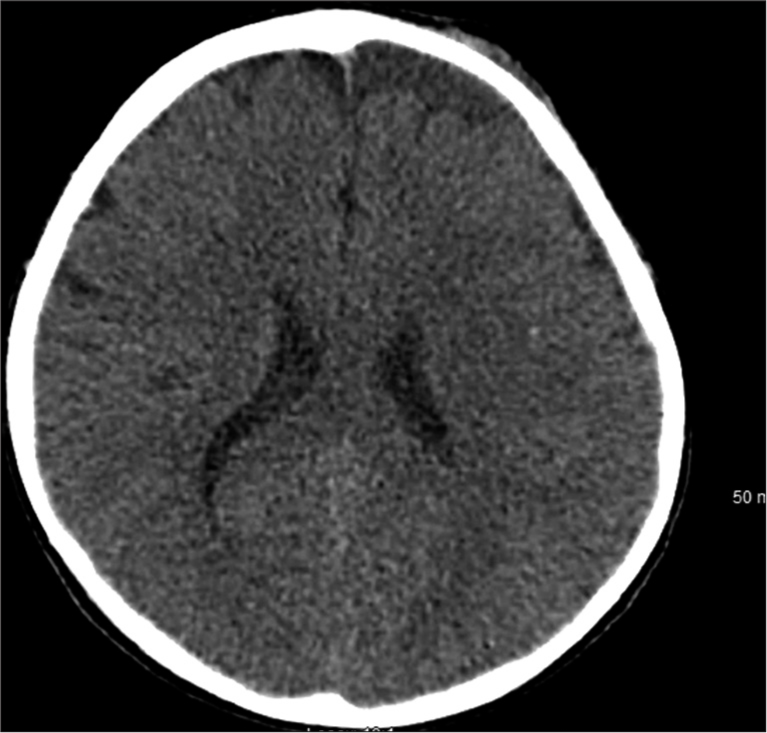
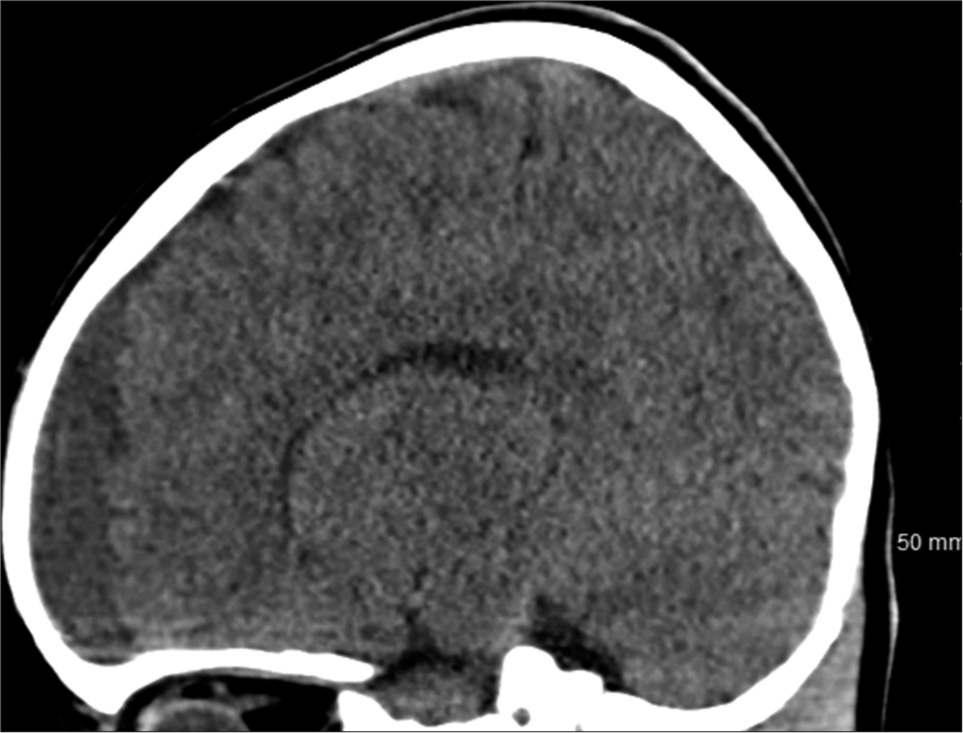
A 3-year-old male with a history of autism along with cognitive, language, and behavioral delays presented to the emergency department after falling 4 feet from a window. Head computerized tomography (CT) revealed a 5 × 5 × 4 cm AC of the left frontal convexity with subjacent mass effect. Before the fall, he had delays in cognitive advancement with difficulty following commands, naming objects, and making eye contact. He was able to ambulate with assistance but unable to climb stairs. He also banged his head against walls frequently while screaming and it was unclear if this was secondary to headaches. The patient received physical, occupational, and behavioral therapy through early intervention therapies with no real improvement. On neurological examination, he made no eye contact, did not follow commands, was not cooperative, did not speak or verbalize, but did demonstrate symmetric movement. The patient’s family stated that he had undergone neuropsychiatric testing which diagnosed autism but the patient’s family did not provide reliable follow-up with us; we were unable to obtain this report. The patient was lost to follow-up for 6 months, and on representation, a magnetic resonance imaging (MRI) was obtained which demonstrated a 5.6 × 5.2 × 4.5 cm left frontal AC with associated mass effect on the adjacent parenchyma and frontal horn of the left lateral ventricle. Ophthalmological assessment revealed no papilledema. The patient underwent a left frontal craniotomy for microsurgical fenestration of the AC with excision of the cyst wall without complication. At 10-day follow-up, his parents reported much calmer behavior with no further labile outbursts and less head banging. At 1 month follow-up, his parents reported significant improvement in cognitive function whereby he was following instructions in computer games and solving multistep problems. His behavior was completely altered and more appropriate for 3 years old and he was making eye contact and interacting with multiple examiners while playing games with them as well. He was following simple commands and no longer banging his head on walls. He was naming objects and persons at home and in clinic. At 3 months follow-up, his behavior continued to improve, most noticeably, with additional verbalization and interaction and eye contact with family and strangers. Unfortunately, the patient was lost to further follow-up [
DISCUSSION
ACs are cerebrospinal fluid containing cysts located between the surface of the brain or spinal cord and arachnoid layer of the meninges. MRI reveals a cyst containing T2 hyperintense fluid and CT reveals hypodense fluid consistent with cerebrospinal fluid. One means of classifying ACs is by location in the cranial vault. For instance, the Galassi classification system is used for ACs in the middle cranial fossa, where they are most commonly located.[
Large AC size leading to localized mass effect (functional parenchymal compression) in combination with their anatomic location is crucial for reconciling whether a patient’s clinical presentation or symptoms localize to the compressed region in determining the next steps in management. Treatment options vary from continued radiographic observation to surgical intervention. Thinning of cortex adjacent to cysts has been observed and studies suggest that these cysts more likely cause reversible suppression of brain function in the area.[
The literature regarding cognitive, language, and behavioral deficits or delays related to ACs in the pediatric population is limited but studies demonstrating improvement in perception and/or memory after surgical treatment of ACs have been documented in adults.[
The few reports that discuss surgical intervention in pediatric patients with ACs have dealt with those of the temporal lobe. One such case reports 6 years old presenting with speech delays and a temporal AC on imaging. A cystoperitoneal shunt was placed with significant language improvement and an increase in patient IQ postoperatively. A second example reports 7 years old presenting with deficits in verbal comprehension and linguistic function with a temporal AC on imaging and significant improvement after shunt placement.[
Limitations of our report include sample size and lack of objective measures such as neuropsychological testing before and after surgery. Our single patient makes it difficult to generalize our findings to the whole of pediatric AC patient population but in conjunction with the larger reported data pool from the adult literature provides further support for surgical decompression. More advanced radiographic studies such as SPECT or PET measure perfusion and metabolism of affected brain regions; demonstrating relative magnitude and distribution of damage. Thus, they may have provided further support for surgery were they to demonstrate widespread findings of cortical impairment adjacent to the AC.[
CONCLUSION
Fenestration of our patient’s left frontal AC resulted in drastic improvements in behavioral, cognitive, and language delays. Additional cases demonstrating behavioral and cognitive improvement in pediatric cases of AC are necessary to devise the best treatment paradigm when traditional surgical indications such as papilledema, rapid growth, or mass effect with midline shift are absent, although anterior fossa ACs compressing frontal lobe remain rare.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
I want to first thank the family and patient described in this case report for allowing us to write about their case to allow increased awareness and education about the treatment of arachnoid cysts.
I want to also thank both Global Neurosciences Institute and Rowan University School of Osteopathic Medicine for allowing the creation and completion of this case.
Finally, I would like to thank all those involved in the revision and submitting process at the Medical College of Wisconsin.
References
1. Choi JW, Lee JY, Phi JH, Kim SK, Wang KC. Stricter indications are recommended for fenestration surgery in intracranial arachnoid cysts of children. Childs Nerv Syst. 2015. 31: 77-86
2. Galassi E, Tognetti F, Gaist G, Fagioli L, Frank F, Frank G. CT scan and medtrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: Classification and pathophysiological aspects. Surg Neurol. 1982. 17: 363-9
3. Horiguchi T, Takeshita K. Cognitive function and language of a child with an arachnoid cyst in the left frontal fossa. World J Biol Psychiatry. 2000. 1: 159-63
4. Jafrani R, Raskin JS, Kaufman A, Lam S. Intracranial arachnoid cysts: Pediatric neurosurgery update. Surg Neurol Int. 2019. 10: 15
5. Kotil K, Kilinc BM, Bilge T. Spinal metastasis of occult lung carcinoma causing cauda equina syndrome. J Clin Neurosci. 2007. 14: 372-5
6. Kwiatkowska K, Hałabuda A, Rybus J, Kwiatkowski S. Cognitive disorders in a patient with an arachnoid cyst of the sylvian fissure and improvement after surgical treatment: Case description. Appl Neuropsychol Child. 2019. 8: 182-6
7. Laporte N, de Volder A, Bonnier C, Raftopoulos C, Sébire G. Language impairment associated with arachnoid cysts: Recovery after surgical treatment. Pediatr Neurol. 2012. 46: 44-7
8. Matsuda W, Akutsu H, Miyamoto S, Noguchi S, Tsunoda T, Sasaki M. Apparently asymptomatic arachnoid cyst: Postoperative improvement of subtle neuropsychological impediment. Neurol Med Chir (Tokyo). 2010. 50: 430-3
9. McDonald PJ, Rutka JT. Middle cranial fossa arachnoid cysts that come and go. Pediatr Neurosurg. 1997. 26: 48-52
10. Mustansir F, Bashir S, Darbar A. Management of arachnoid cysts: A comprehensive review. Cureus. 2018. 10: e2458
11. Raeder MB, Helland CA, Hugdahl K, Wester K. Arachnoid cysts cause cognitive deficits that improve after surgery. Neurology. 2005. 64: 160-2
12. Torgersen J, Helland C, Flaatten H, Wester K. Reversible dyscognition in patients with a unilateral, middle fossa arachnoid cyst revealed by using a laptop based neuropsychological test battery (CANTAB). J Neurol. 2010. 257: 1909-16
13. Tsurushima H, Harakuni T, Saito A, Tominaga D, Hyodo A, Yoshii Y. Symptomatic arachnoid cyst of the left frontal convexity presenting with memory disturbance. Neurol Med Chir (Tokyo). 2000. 40: 339-41
14. Wester K. Intracranial arachnoid cysts-do they impair mental functions?. J Neurol. 2008. 255: 1113
15. Wester K, Hugdahl K. Arachnoid cysts of the left temporal fossa: Impaired preoperative cognition and postoperative improvement. J Neurol Neurosurg Psychiatry. 1995. 59: 293-8
16. Yamauchi T, Saeki N, Yamaura A. Spontaneous disappearance of temporo-frontal arachnoid cyst in a child. Acta Neurochir (Wien). 1999. 141: 537-40
17. Zaatreh MM, Bates ER, Hooper SR, Palmer G, Elmenshawi EE, Courvoisie HE. Morphometric and neuropsychologic studies in children with arachnoid cysts. Pediatr Neurol. 2002. 26: 134-8