- Department of Neurosurgery, Shimoishibikimachi, Kanazawa, Ishikawa, Japan.
- Department of Neurosurgery, Kanazawa Medical Center, Shimoishibikimachi, Kanazawa, Ishikawa, Japan.
Correspondence Address:
Yu Shimizu
Department of Neurosurgery, Kanazawa Medical Center, Shimoishibikimachi, Kanazawa, Ishikawa, Japan.
DOI:10.25259/SNI_578_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yu Shimizu, Katsuhiro Tsuchiya, Hironori Fujisawa. Reversible cerebral vasoconstriction syndrome concomitant with cerebral venous sinus thrombosis following ovarian tumor resection: A report of two cases. 30-May-2020;11:128
How to cite this URL: Yu Shimizu, Katsuhiro Tsuchiya, Hironori Fujisawa. Reversible cerebral vasoconstriction syndrome concomitant with cerebral venous sinus thrombosis following ovarian tumor resection: A report of two cases. 30-May-2020;11:128. Available from: https://surgicalneurologyint.com/surgicalint-articles/10054/
Abstract
Background: Reversible cerebral vasoconstriction syndrome (RCVS) presents with characteristic clinical, brain imaging, and angiographic findings. The most common clinical feature of RCVS is a severe acute headache, which is often referred to as a thunderclap headache due to the nature of its presentation. It may occur spontaneously or may be provoked by various precipitating factors. We present a rare case of RCVS concomitant with cerebral venous sinus thrombosis (CVST) in a woman who underwent resection of an ovarian tumor.
Case Description: Case 1 – A 42-year-old woman was admitted to our hospital with severe headache radiating to the neck, with associated vomiting. She revealed a medical history of ovarian cancer and underwent an operation for the resection of the tumor, a month before presentation. After resection, her estradiol (E2) levels were reduced from 288 pg/ml to 31 pg/ml (normal range, 0–49 pg/ml). Initial imaging on admission to our hospital revealed the left posterior convexity subarachnoid hemorrhage. Magnetic resonance angiography (MRA) showed findings consistent with RCVS affecting the left posterior cerebral artery. Magnetic resonance venography (MRV) showed CVST of the left transverse and sigmoid sinuses. Single-photon emission computed tomography (SPECT) showed a left posterior ischemic lesion. These findings improved following treatment with nimodipine and anticoagulant. Case 2 – A 39-year-old woman presented with holocranial headache associated with vomiting. She was diagnosed with an ovarian tumor. She underwent an operation 3 months before presentation. After tumor resection, her E2 level decrease from 193 pg/ml to 19 pg/ml (normal range, 0–49 pg/ml). Magnetic resonance angiography (MRA) confirmed the presence of a vasospasm involving the right anterior cerebral artery. Magnetic resonance venography (MRV) confirmed the presence of thrombosis involving the superior sagittal sinus. She was discharged on postpartum day 31 without neurological deficits after treatment with anticoagulants. At her 3-month follow-up, both MRA and MRV were within the normal limits.
Conclusion: This is the first report of two women diagnosed with RCVS with concomitant CVST following ovarian tumor resection. Marked reductions in postoperative E2 levels could have contributed to the development of CVST and RCVS.
Keywords: Reversible cerebral vasoconstriction syndrome, Cerebral venous sinus thrombosis, Ovarian tumor, Single-photon emission computed tomography, Edoxaban
INTRODUCTION
Reversible cerebral vasoconstriction syndrome (RCVS) is a unifying termused to describe a group of disorders sharing angiographic and clinical features, such as reversible segmental and multifocal vasoconstriction of cerebral arteries and severe headaches with or without focal neurological deficits or seizures.[
CASE DESCRIPTION
Case 1
A 42-year-old woman presented with a 3-week history of headache showing recent progression, as well as disorientation and reduced level of consciousness. She revealed a medical history of ovarian tumor resection [
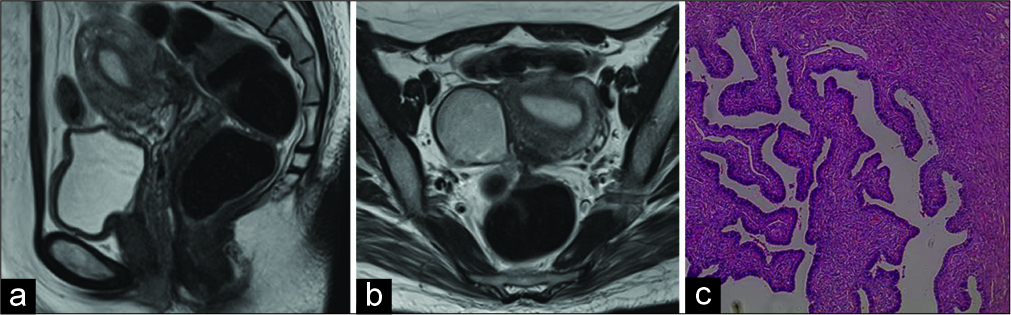
Figure 1:
(a,b) Sagittal and axial T2-weighted MR image shows a large cystic ovarian tumor of 5cm at maximum diameter. The tumor had arisen from the right ovary, the margin was smooth and the uterus was normal size. (c) Ovarian endometrioid tumor of low malignant potential showing glands similar to the complex hyperplasia of the uterine endometrium.
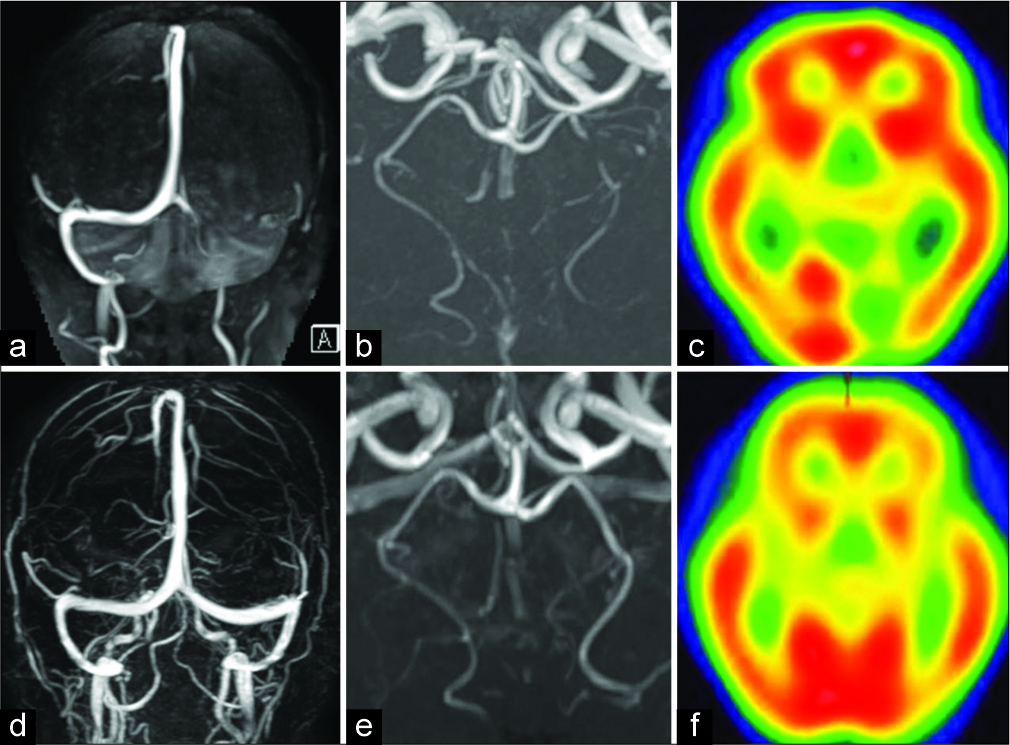
Figure 2:
(a) Brain magnetic resonance venography image obtained on admission showing occlusion of the left transverse and sigmoid sinuses. Magnetic resonance venography image obtained 28 days after admission showing recanalization of the venous sinus (d). (b) 3D TOF MRA showing high-grade left PCA stenosis. Improvement in vasoconstriction is observed on day 14 from ictus (e). (c) SPECT images obtained on day 2 from ictus showing left posterior ischemic lesion. SPECT image obtained on day 28 from ictus showing absence of the ischemic lesion (f).
Her MRV revealed no abnormalities a month later [
Case 2
A 39-year-old female with a recent history of ovarian tumor resection developed holocranial headache associated with vomiting. After tumor resection, her E2 level decreased from 193 pg/ml to 19 pg/ml (normal range, 0–49 pg/ml). Neurological examination revealed no focal neurologic deficits. The next day she experienced repeat onset of severe headache followed by right lower limb paresis. On presentation to our department, she underwent a clinical examination which revealed all vital signs to be within the normal ranges. Magnetic resonance angiography (MRA) confirmed the presence of a vasospasm involving the right anterior cerebral artery [
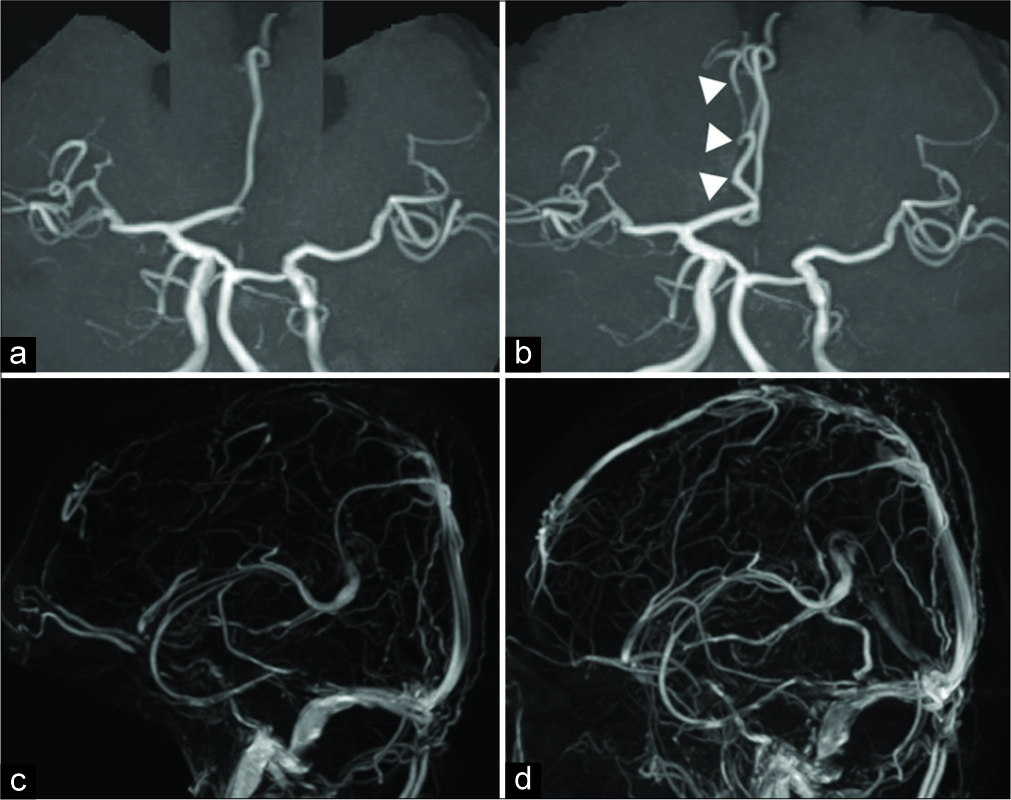
Figure 3:
(a,c) Magnetic resonance angiography (MRA) confirmed the presence of a vasospasm involving the right anterior cerebral artery. On admission, MRA revealed vasoconstriction of the anterior cerebral artery. Vasoconstriction was normalized within 3 months. (b,d) Magnetic resonance venography (MRV) confirmed the presence of a thrombosis involving the superior sagittal sinus. On admission, MRV revealed thrombosis of the superior sagittal sinus, which normalized within 3 months.
DISCUSSION
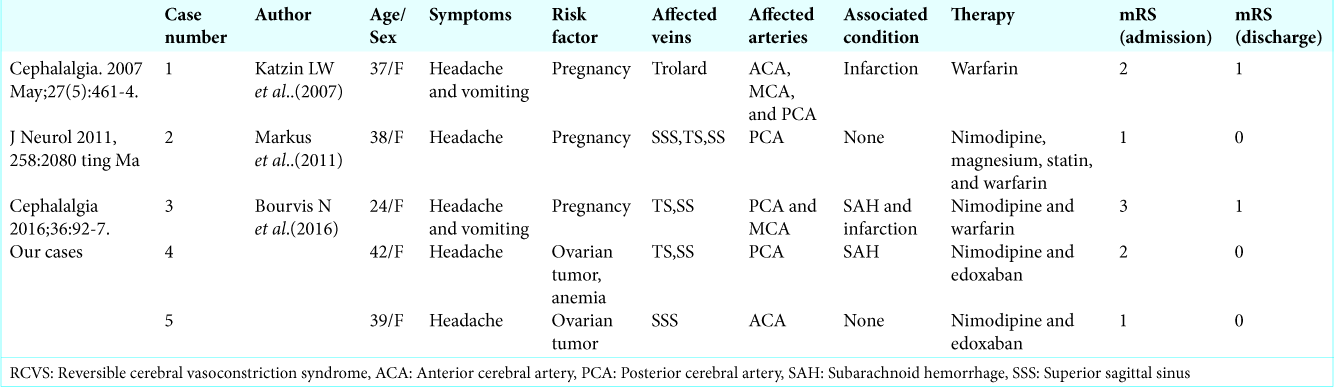
Cerebral venous thrombosis (CVT) is an uncommon form of stroke that presents with a wide range of clinical manifestations. Risk factors include ovarian tumor, iron deficiency anemia, pregnancy, intravenous drug abuse, infection, and dehydration.[
The following pathomechanisms should be considered in the present case: RCVS and CVT coexisted in this woman or CVT resulted in RCVS. An association between CVT and RCVS has previously been reported in two women immediately postpartum.[
CONCLUSION
Our cases highlight that both RCVS and CVST should be considered perioperatively in women undergoing ovarian tumor resection. In our patients, resection of the ovarian tumor led to dramatically reduced E2 levels, which could have contributed to the development of CVST and RCVS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bourvis N, Franc J, Szatmary Z, Chabriat H, Crassard I, Ducros A. Reversible cerebral vasoconstriction syndrome in the context of recent cerebral venous thrombosis: Report of a case. Cephalalgia. 2016. 36: 92-7
2. Bousser MG. Antithrombotic strategy in stroke. Thromb Haemost. 2001. 86: 1-7
3. Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: Reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007. 146: 34-44
4. Dash D, Prasad K, Joseph L. Cerebral venous thrombosis: An Indian perspective. Neurol India. 2015. 63: 318-28
5. Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007. 130: 3091-101
6. Freilinger T, Schmidt C, Duering M, Linn J, Straube A, Peters N. Reversible cerebral vasoconstriction syndrome associated with hormone therapy for intrauterine insemination. Cephalalgia. 2010. 30: 1127-32
7. Hoeren M, Hader C, Strümpell S, Weiller C, Reinhard M. Peripartum angiopathy with simultaneous sinus venous thrombosis, cervical artery dissection and cerebral arterial vasoconstriction. J Neurol. 2011. 258: 2080-2
8. Matano F, Murai Y, Adachi K, Koketsu K, Kitamura T, Teramoto A. Reversible cerebral vasoconstriction syndrome associated with subarachnoid hemorrhage triggered by hydroxyzine pamoate. Clin Neurol Neurosurg. 2013. 115: 2189-91
9. Robert T, Oumarou G, Villard J, Marchini AK, Daniel RT, Levivier M. Call-Fleming syndrome associated with convexity subarachnoid hemorrhage. Clin Neurol Neurosurg. 2013. 115: 796-8
10. Silvis SM, Middeldorp S, Zuurbier SM, Cannegieter SC, Coutinho JM. Risk factors for cerebral venous thrombosis. Semin Thromb Hemost. 2016. 42: 622-31
11. Singhal AB, Bernstein RA. Postpartum angiopathy and other cerebral vasoconstriction syndromes. Neurocrit Care. 2005. 3: 91-7
12. Soo Y, Singhal AB, Leung T, Yu S, Mak H, Hao Q. Reversible cerebral vasoconstriction syndrome with posterior leucoencephalopathy after oral contraceptive pills. Cephalalgia. 2010. 30: 42-5