- Department of Surgical Neurology Research Institute for Brain and Blood Vessels-Akita, Akita, Japan.
- Department of Radiology and Nuclear Medicine, Research Institute for Brain and Blood Vessels-Akita, Akita, Japan.
Correspondence Address:
Kohei Yoshikawa, Department of Surgical Neurology, Research Institute for Brain and Blood Vessels-Akita, Akita, Japan.
DOI:10.25259/SNI_618_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kohei Yoshikawa1, Junta Moroi1, Kohei Kokubun1, Nobuharu Furuya1, Yasuyuki Yoshida1, Toshibumi Kinoshita2, Yuki Shinohara2, Tatsuya Ishikawa1. Role of magnetic resonance vessel wall imaging in detecting and managing ruptured aneurysms among multiple intracranial aneurysms. 13-Sep-2021;12:460
How to cite this URL: Kohei Yoshikawa1, Junta Moroi1, Kohei Kokubun1, Nobuharu Furuya1, Yasuyuki Yoshida1, Toshibumi Kinoshita2, Yuki Shinohara2, Tatsuya Ishikawa1. Role of magnetic resonance vessel wall imaging in detecting and managing ruptured aneurysms among multiple intracranial aneurysms. 13-Sep-2021;12:460. Available from: https://surgicalneurologyint.com/surgicalint-articles/11111/
Abstract
Background: Wall enhancement of intracranial saccular aneurysms in high-resolution magnetic resonance vessel wall imaging (MR-VWI) might indicate a ruptured aneurysm. Therefore, this study aimed to determine the diagnostic ability of wall enhancement to detect the ruptured aneurysms among multiple aneurysms.
Methods: Patients with subarachnoid hemorrhage (SAH) and multiple intracranial aneurysms who underwent MR-VWI before craniotomy and clipping were included in the study. Three-dimensional T1-weighted fast spin-echo sequences were obtained before and after gadolinium injection. Aneurysm rupture was estimated based on the subarachnoid clot distribution, aneurysmal contours, and MR-VWI findings. We selectively performed surgical clipping and confirmed the rupture site intraoperatively.
Results: Thirteen patients with SAH with 13 ruptured and 17 unruptured aneurysms were treated at out facility. The accuracy rate of rupture site diagnosis using MR-VWI was 69.2% (9/13 cases). Each unruptured aneurysm was equally or more strongly enhanced in the other four cases than the ruptured aneurysms. In three of the four unruptured aneurysms with positive MR-VWI findings, atherosclerosis of the aneurysmal wall was observed during simultaneous or elective clipping surgery. Further, clipping surgery was performed without intraoperative rupture in two cases with the help of MR-VWI findings.
Conclusion: Correct diagnosis of the rupture site using MR-VWI alone was unreliable due to false positives caused by the wall enhancement of unruptured aneurysms with atherosclerosis. Therefore, ruptured aneurysms should be detected using more information in addition to MR-VWI images. MR-VWI may be advantageous to determine surgical strategies when managing patients with SAH and multiple aneurysms.
Keywords: Clipping surgery, Magnetic resonance-vessel wall imaging, Multiple aneurysms, Ruptured cerebral aneurysm
INTRODUCTION
Recent studies have suggested that wall enhancement of intracranial saccular aneurysms in high-resolution magnetic resonance vessel wall imaging (MR-VWI) might be an indicator of aneurysm rupture and the rupture site.[
Information regarding the rupture site before surgery might be essential to establish the operative strategy and safely perform the procedure. However, the advantages of performing MR-VWI in conjunction with clipping surgery have not yet been reported.
In this study, we report our findings of the diagnostic accuracy rate of MR-VWI and discuss the clinical advantages of this technique.
MATERIALS AND METHODS
Preoperative MR-VWI protocol
MR-VWI was performed on a 3T MRI scanner (Verio or Skyra, SIEMENS, Erlangen, Germany) and comprised a T1-weighted black-blood three-dimensional (3D) volume isotropic fast spin-echo (FSE) acquisition sequence. The imaging parameters were echo time/repetition time: 20/600 ms; matrix: 256 × 256; field of view: 190 × 190 mm; voxel size: 0.4 × 0.4 × 0.5 mm; and, acquisition time: 5 min 41 s. 3D T1-weighted FSE sequences were obtained before and after intravenous contrast injections of a dose (0.1 mmol/kg) of gadobutrol (Gadovist; Bayer, Leverkusen, Germany). The images were evaluated by two neurosurgeons and two experienced neuroradiologists. The enhancements were classified as “strong,” “faint,” and “no” enhancement (strong enhancement: definite enhancement equal to that of the choroid or venous plexus; faint enhancement: increased signal intensity of wall compared to the precontrast scan) according to Nagahata et al.[
Patient population
Our study included patients with SAH and multiple intracranial saccular aneurysms who had undergone MR-VWI before craniotomy and clipping surgery at our institute between January 1, 2016, and December 31, 2020. We evaluated the aneurysms that appeared to be ruptured before surgery based on hemorrhage distribution, aneurysmal shape or size, and MR-VWI findings to establish the treatment strategy. After clipping surgery, the modified Rankin scale (mRS) score was determined during hospital discharge, and surgical complications, including intraoperative ruptures, were used as outcome measures.
All study participants provided informed consent, and the appropriate ethics review board approved the study design.
Surgical procedures
Distal anterior cerebral artery (ACA) and anterior communicating artery (A-com) aneurysms were usually treated through the basal interhemispheric approach.[
RESULTS
Thirteen patients with SAH had 13 ruptured and 17 unruptured aneurysms. The mean age was 64.1 years (range 33–83 years). The mean size of the ruptured aneurysms was 5.5 mm (range 1.5–11 mm) and the median time from rupture to surgery was 21.5 h (range 3.5–336 h). All 13 ruptured aneurysms had wall enhancement; conversely, this enhancement was only seen in five (29.4%) unruptured aneurysms. Strong and faint enhancement was seen in nine (69.2%) and four (30.8%) of ruptured aneurysms, and three (17.6%) and two (11.8%) of unruptured aneurysms, respectively.
Nine ruptured aneurysms (69.2%) had the greatest wall enhancement among the coexisting aneurysms. The other four ruptured aneurysms (30.8%) did not have a greater enhancement than each enhanced unruptured aneurysm. In three of the five unruptured aneurysms with positive MR-VWI, atherosclerosis of the aneurysmal wall was observed during simultaneous or elective clipping surgery.
One patient (7.7%) had an intraoperative rupture, and the other four patients experienced surgical complications: one case of anosmia, two asymptomatic infarctions, and one symptomatic infarction. In total, 11 patients (84.6%) had a favorable outcome (mRS: 0–2) at hospital discharge [
We concluded that the patient-based diagnostic accuracy rate of ruptured aneurysms using MR-VWI was 69.2% (9/13 cases). The aneurysm-based sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of MR-VWI in predicting aneurysm rupture are shown in [
Case presentations
Case 1: Multiple aneurysms in a single approach route
A 66-year-old female presented with a persistent headache. Initial 3D computed tomography angiography (3D-CTA) 12 days after the headache onset showed a thin subarachnoid clot in the interhemispheric fissure and two bilateral ACA aneurysms [
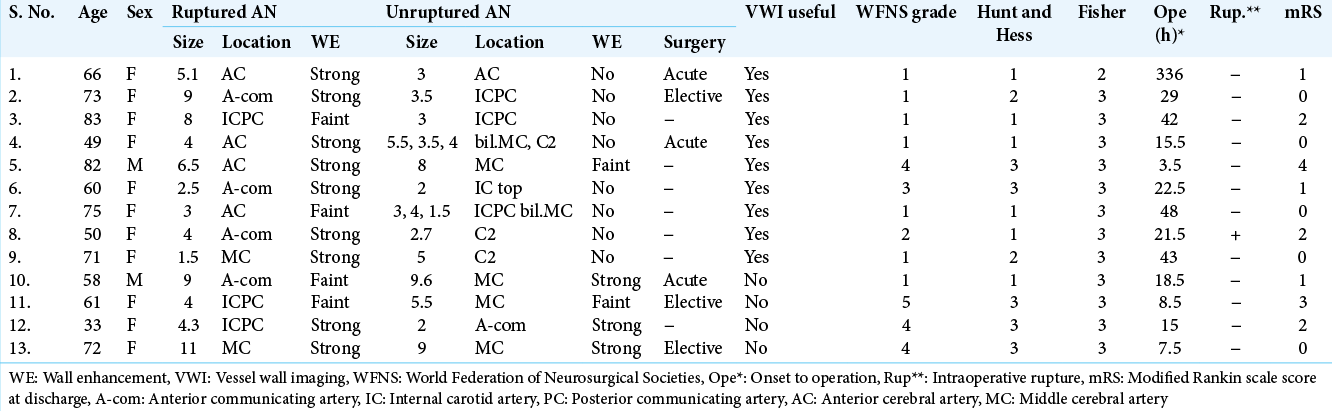
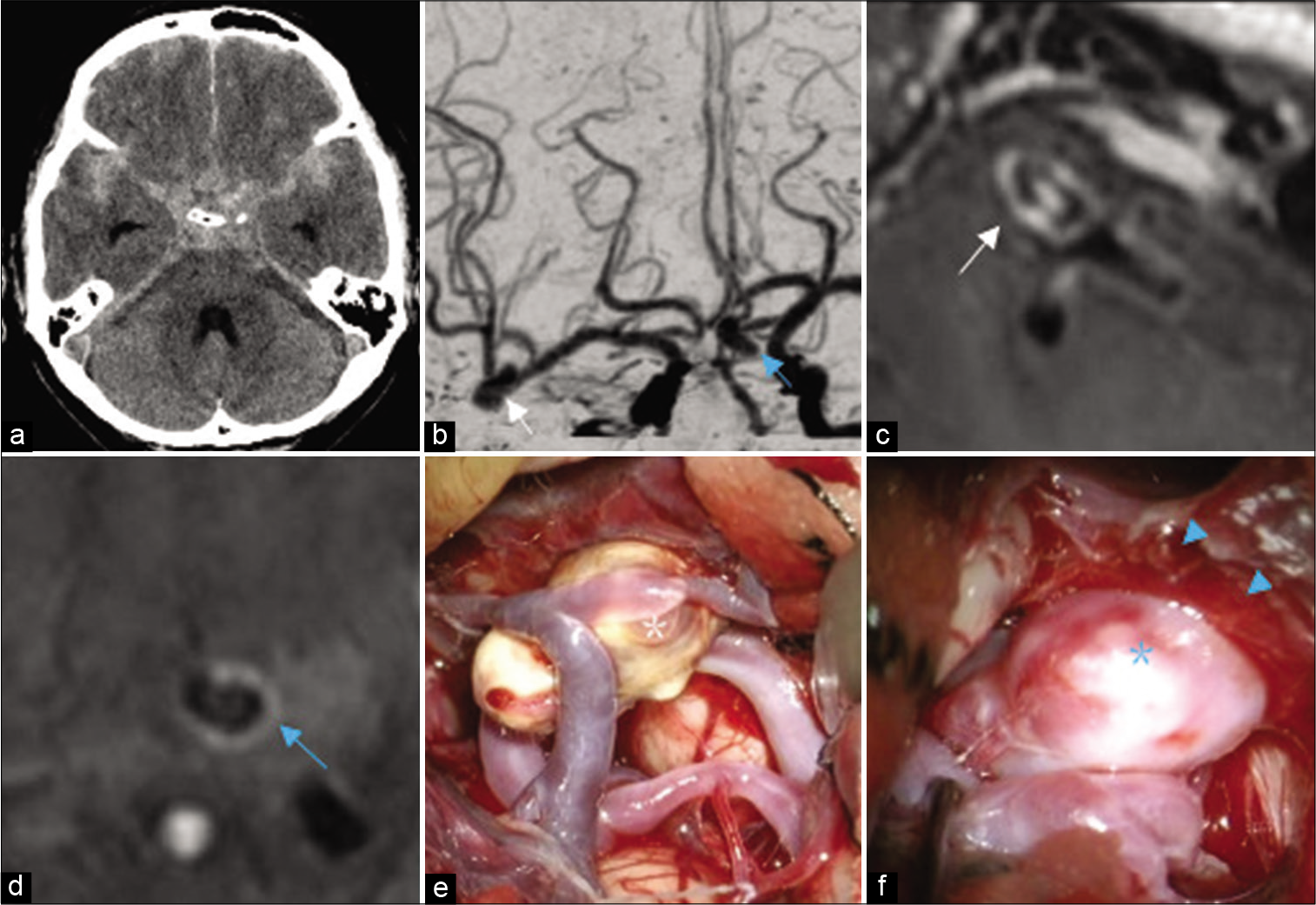
Figure 1:
Multiple aneurysms in a single approach route. Case 1. A 66-year-old female underwent clipping surgery for bilateral distal anterior cerebral artery (ACA) aneurysms. (a) Computed tomography (CT) image shows a thin subarachnoid clot in the interhemispheric fissure. (b and c) Three-dimensional CT angiography and magnetic resonance-vessel wall imaging shows bilateral ACA aneurysms. The left ACA aneurysm has a more irregular shape and stronger wall enhancement (arrowhead) and rupture is suspected. (d) At first, the right ACA aneurysm in front of the surgical field (green asterisk) was checked and found to be unruptured during surgery. (e) Next, we were able to expose the proximal artery (left A2; arrow) and the neck of the left ACA aeurysm (blue asterisk) widely without focusing on the right ACA aneurysm. (f) After clipping, the top of the aneurysm that is covered with a hemostatic clot (double arrow) is confirmed to be the rupture point.
We first reached proximal to the deeply located left aneurysm without focusing on the right aneurysm [
Case 2: Multiple aneurysms, including a multi-lobulated aneurysm
A 73-year-old female with complaints of acute headache was found to have a distributed subarachnoid clot predominantly on the right side of the initial CT scan on day 0 [
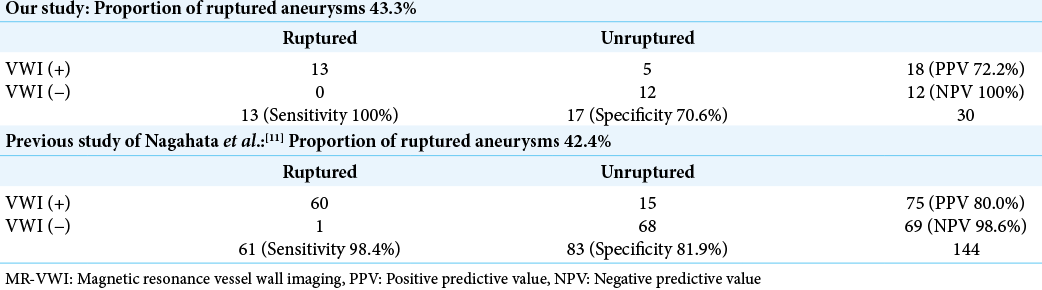
Figure 2:
Multiple aneurysms, including a multi-lobulated aneurysm. Case 2. A 73-year-old female underwent clipping surgery for a multi-lobulated anterior communicating artery (A-com) aneurysm. (a) Computed tomography (CT) image reveals a subarachnoid clot predominantly on the right side. (b and c) Three-dimensional CT angiography image shows a multi-lobulated A-com and a right internal carotid artery aneurysm. The right side of the A-com aneurysm (blue arrow) has strong wall enhancement and is estimated to be the rupture point, but the lower and left side (green arrow) has no enhancement on magnetic resonance-vessel wall imaging. (d and e) Intraoperative view through the basal interhemispheric approach. At first, we approached the safer left side and were able to expose the proximal artery (left A1; white arrow) and the left neck near the left bleb (green arrowhead). (f) Next, we exposed the right neck carefully and clipped the multi-lobulated A-com aneurysm in a lump. The right side of the aneurysm (blue asterisk) with vessel wall enhancement is composed of a very thin wall and has the rupture point covered with a hemostatic clot.
The A-com aneurysm had a multi-lobulated shape with a bleb on the left side, and it was difficult to identify the rupture point solely based on 3D-CTA findings. In this case, however, we were able to presume that the rupture point would be located on the right side of the aneurysm, based on the strong enhancement on the MR-VWI [
Case 10: Difficulty in identifying the ruptured aneurysm
A 58-year-old male presenting with acute headache was found to have SAH, a right middle cerebral artery (MCA), and an A-com aneurysm on 3D-CTA image on day 0 [
Figure 3:
Difficulty in identifying the ruptured aneurysm. Case 10. A 58-year-old male underwent clipping surgery for a right middle cerebral artery (MCA) and an anterior communicating artery (A-com) aneurysm. (a) Computed tomography (CT) image shows a diffuse subarachnoid clot. (b) Three-dimensional CT angiography image demonstrates the two aneurysms of the right MCA (white arrow) and the A-com (blue arrow). (c and d) Both aneurysms have wall enhancement on magnetic resonance-vessel wall imaging (MR-VWI) (right MCA: white arrow indicates strong wall enhancement and enhancement in the lumen; A-com: blue arrow indicates faint wall enhancement). (e) Intraoperative photograph using a right transsylvian approach in order to treat both aneurysms simultaneously. The MCA aneurysm (white asterisk) is found to have a circumferential atherosclerotic unruptured wall. (f) The A-com aneurysm (blue asterisk) is covered with a hemostatic clot (blue arrowhead) and is presumed to be ruptured. Both aneurysms were clipped and complete hemostatic surgery was performed without relying on MR-VWI data.
DISCUSSION
In patients with SAH and multiple intracranial aneurysms, surgeons can often misdiagnose a ruptured aneurysm as an unruptured aneurysm and leave it untreated. This diagnosis may result in a fatal aneurysm rupture. However, it is not always easy to distinguish a ruptured aneurysm from coexisting unruptured aneurysms.
In the present study, we have determined the diagnostic accuracy rate of MR-VWI in predicting ruptured aneurysms. Our findings indicate that while MR-VWI may be useful in determining surgical strategies in handling patients with SAH, it must be combined with other methods to detect aneurysm successfully and cannot be a stand-alone technique.
In 1985, Nehls et al. reported that 93.3% of aneurysms with irregular shape and 83.3% of larger aneurysms were ruptured. However, the diagnostic utility of subarachnoid clot distribution on a CT scan as a biomarker was seen in only 45% of cases. The ruptured and unruptured aneurysms were either equal or inconsistent in size and shape in 24.4% of cases.[
Recently, it has been reported that the wall enhancement of cerebral aneurysms on MRI provides additional information for detecting the location of aneurysm rupture. Nagahata et al. found strong and faint enhancement on the MR-VWI in 73.8% and 24.6% of ruptured aneurysms and 4.8% and 13.3% of unruptured aneurysms, respectively.[
As a note of MR-VWI interpretation, smaller aneurysms tend to have weak enhancement even in a 3T-MRI study,[
From a technical point of view, MR-VWI enables us to strategically select the proper approach route and surgical procedure to manage ruptured aneurysms when there are multiple intracranial aneurysms. For example, in the case ten patient, two aneurysms, both of which had an indistinguishable wall enhancement but were simple in shape, were treated simultaneously using a single approach. On the other hand, in the case 2 patient , basal interhemispheric approach was selected to accommodate a complex-shaped A-com aneurysm.[
The incidence of intraoperative rupture during clipping surgery for ruptured aneurysms has been reported to be 14.3–20%;[
The aneurysms that were preoperatively estimated to be unruptured using MR-VWI did not rupture. None of our patients experienced serious surgical complications, and the outcomes were generally favorable (mRS: 0-2) at discharge.
Craniotomy and MR-VWI combine well since they allow for the aneurysm rupture to be confirmed with greater certainty than preoperative observation, and further treatment can be added on when necessary. However, when performing endovascular treatment for patients with SAH and multiple aneurysms, MR-VWI findings should be interpreted very carefully when we selectively coil one targeted aneurysm among multiple aneurysms since it is not possible to confirm beforehand that if the targeted aneurysm is indeed ruptured or not. It should be remembered that positive MR-VWI findings indicate surgery, but negative MR-VWI findings cannot preclude surgery. Simultaneous coiling of multiple aneurysms in a single endovascular surgery session is necessary in such cases.
The limitations of this study should be discussed. It should be noted that this was a retrospective study with a small number of patients and the single-center study. In addition, there was a selection bias since cases treated with endovascular surgery were excluded from this study, and comprehensive judgment of the ruptured aneurysm and selection of the approach route is subjective to some extent. Future research is needed on how MR-VWI influences clinical decisions and what results are produced.
CONCLUSION
The diagnostic accuracy rate of aneurysm rupture using MR-VWI alone is unreliable due to false positives caused by the enhancement of unruptured aneurysms with atherosclerosis. Therefore, ruptured aneurysms should be confirmed using additional information, especially if we intend to treat them selectively. However, MR-VWI may provide a technical advantage in determining the surgical strategy and deciding on the optimal operative procedures to manage patients with SAH and multiple aneurysms. We expect that this small study will lead to more routine use of multiple modalities to determine aneurysm rupture and to further studies on MR-VWI interpretation and clinical application.
Human and animal rights
All reported studies involving human participants and procedures performed in studies were in accordance with applicable ethical standards of the institution and/or institutional guidelines, and the 1975 Helsinki Declaration and its later amendments or comparable ethical standards.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Human and animal rights
All reported studies involving human participants and procedures performed in studies were in accordance with applicable ethical standards of the institution and/or institutional guidelines, and the 1975 Helsinki Declaration and its later amendments or comparable ethical standards.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
1. Aoki S, Shirouzu I, Sasaki Y, Okubo T, Hayashi N, Machida T. Enhancement of the intracranial arterial wall at MR imaging: relationship to cerebral atherosclerosis. Radiology. 1995. 194: 477-81
2. Batjer H, Samson D. Intraoperative aneurysmal rupture: Incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986. 18: 701-7
3. Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999. 45: 1137-46
4. Edjlali M, Gentric JC, Régent-Rodriguez C, Trystram D, Ben Hassen W, Lion S. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms?. Stroke. 2014. 45: 3704-6
5. Endo H, Niizuma K, Fujimura M, Sato K, Inoue T, Osawa SI. Ruptured cerebral microaneurysm diagnosed by 3-dimensional fast spin-echo T1 imaging with variable flip angles. J Stroke Cerebrovasc Dis. 2015. 24: e231-5
6. Giannotta SL, Oppenheimer JH, Levy ML, Zelman V. Management of intraoperative rupture of aneurysm without hypertension. Neurosurgery. 1991. 28: 531-6
7. Ishikawa T, Nakayama N, Yoshimoto T, Aoki T, Terasaka S, Nomura M. How does spontaneous hemostasis occur in ruptured cerebral aneurysms? Preliminary investigation on 247 clipping surgeries. Surg Neurol. 2006. 66: 269-76
8. Jomin M, Lesoin F, Lozes G. Prognosis with 500 ruptured and operated intracranial arterial aneurysms. Surg Neurol. 1984. 21: 13-8
9. Larsen N, von der Brelie C, Trick D, Riedel CH, Lindner T, Madjidyar J. Vessel wall enhancement in unruptured intracranial aneurysms: An indicator for higher risk of rupture? High resolution MR imaging and correlated histologic findings. AJNR Am J Neuroradiol. 2018. 39: 1617-21
10. Matouk CC, Mandell DM, Günel M, Bulsara KR, Malhotra A, Hebert R. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: Proof of principle. Neurosurgery. 2013. 72: 492-6
11. Nagahata S, Nagahata M, Obara M, Kondo R, Minagawa N, Sato S. Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: A sign of ruptured aneurysm?. Clin Neuroradiol. 2016. 26: 277-83
12. Nehls DG, Flom RA, Carter PL, Spetzler RF. Multiple intracranial aneurysms: Determining the site of rupture. J Neurosurg. 1995. 63: 342-8
13. Quan K, Song J, Yang Z, Wang D, An Q, Huang L. Validation of wall enhancement as a new imaging biomarker of unruptured cerebral aneurysm. Stroke. 2019. 50: 1570-3
14. Wan X, Zhu C, Leng Y, Degnan AJ, Lu J. Intracranial aneurysm wall enhancement associated with aneurysm rupture: A systematic review and meta-analysis. Acad Radiol. 2019. 26: 664-73
15. Yasui N, Nathal E, Fujiwara H, Suzuki A. The basal interhemispheric approach for acute anterior communicating aneurysms. Acta Neurochir (Wien). 1992. 118: 91-7