- Department of Medicine, Albert Einstein Israeli Faculty of Health Sciences, São Paulo, Brazil.
- Department of Medicine, Catanduva Medical School (FAMECAUNIFIPA), Catanduva, Brazil.
- Department of Medicine, Santa Casa de Sao Paulo School of Medical Sciences, Sao Paulo, Brazil.
- Department of Medicine, São Leopoldo Mandic, Campinas, Brazil.
- Department of Neurosurgery, Hospital Saúde de Caxias do Sul, Caxias do Sul, Brazil.
- Department of Neurology, Pontifical Catholic University of São Paulo, Sorocaba, Brazil.
DOI:10.25259/SNI_918_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rafael Trindade Tatit1, Paulo Eduardo Albuquerque Zito Raffa2, Giovana Cassia de Almeida Motta3, André Alexandre Bocchi4, Júlia Loripe Guimaraes1, Paulo Roberto Franceschini5, Paulo Henrique Pires de Aguiar6. Rosai-Dorfman disease mimicking images of meningiomas: Two case reports and literature review. 21-Jun-2021;12:292
How to cite this URL: Rafael Trindade Tatit1, Paulo Eduardo Albuquerque Zito Raffa2, Giovana Cassia de Almeida Motta3, André Alexandre Bocchi4, Júlia Loripe Guimaraes1, Paulo Roberto Franceschini5, Paulo Henrique Pires de Aguiar6. Rosai-Dorfman disease mimicking images of meningiomas: Two case reports and literature review. 21-Jun-2021;12:292. Available from: https://surgicalneurologyint.com/surgicalint-articles/10905/
Abstract
Background: Rosai-Dorfman disease (RDD) is a rare non-Langerhans cell histiocytic proliferative disorder classically as a massive cervical lymphadenopathy. However, over the years, extranodal locations were confirmed with the central nervous system involvement in less than 5% of cases, which is marked as a significant differential diagnosis of meningiomas, with which they are widely confused due to the similarity of their radiological images.
Case Description: We report a 37-year-old man and 45-year-old man who were diagnosed with intracranial RDD but whose radiological images mimic meningiomas, requiring anatomopathological and tumor’s immunohistochemistry for definitive diagnosis. Moreover, a review of 184 publications with 285 cases of intracranial involvement of this disease was also performed, comparing these findings with those brought in the previous studies.
Conclusion: Intracranial Rosai-Dorfman tumors should always be remembered as differential diagnosis of meningiomas since they are similar radiologically and macroscopically. Once remembered and diagnosed, the lesion must be treated following the same pattern of resection done in meningiomas and, treatment’s differences will not occur in the surgical excision technique, but in complementary chemotherapy implementation, radiotherapy, and even with radiosurgery aid, depending on the case. Thus, it is possible to obtain better results than with just the isolated surgical procedure.
Keywords: Central nervous system, Histiocytosis, Magnetic resonance imaging, Meningioma, Rosai-Dorfman disease
INTRODUCTION
Rosai-Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis characterized by accumulation of activated histiocytes in the affected tissues. Widely heterogeneous and with a variety of clinical phenotypes, it may be present from the isolated form to the form in association with other diseases such as autoimmune,[
His first description dates back to 1965 by a French pathologist - Pierre Paul Louis Lucien Destombes,[
In intracranial RDD, the most involved structures are the suprasellar region, cerebral convexity, parasagittal region, cavernous sinus, and petroclival region,[
The present study reports two cases of RDD with intracranial involvement, one of them with follow-up of more than 15 years. A review of 184 publications with 285 cases of RDD with CNS involvement (CNS-RDD) was also performed, comparing these findings with those brought in the previous studies. For identifying the studies, the MeSH tool from PubMed database was used, using the keywords “Histiocytosis, Sinus” restrict to MeSH Major Topic (entry terms: histiocytoses, Sinus; Sinus Histiocytoses; Sinus Histiocytosis; RDD; Disease, Rosai-Dorfman; RDD; Sinus Histiocytosis with Massive Lymphadenopathy; DestombesRosai-Dorfman Syndrome; Destombes Rosai Dorfman Syndrome; and Syndrome, Destombes-Rosai-Dorfman) and the keyword “Central Nervous System;” no filter was used for languages, date of publication or type of study. In addition, manual searches were performed based on the studies found by the initial electronic search. All articles including new cases of the disease and containing basic information (sex, age, location of the pathology, and if there was isolated involvement of the CNS) were included in the study.
CASES REPORTS
First case report
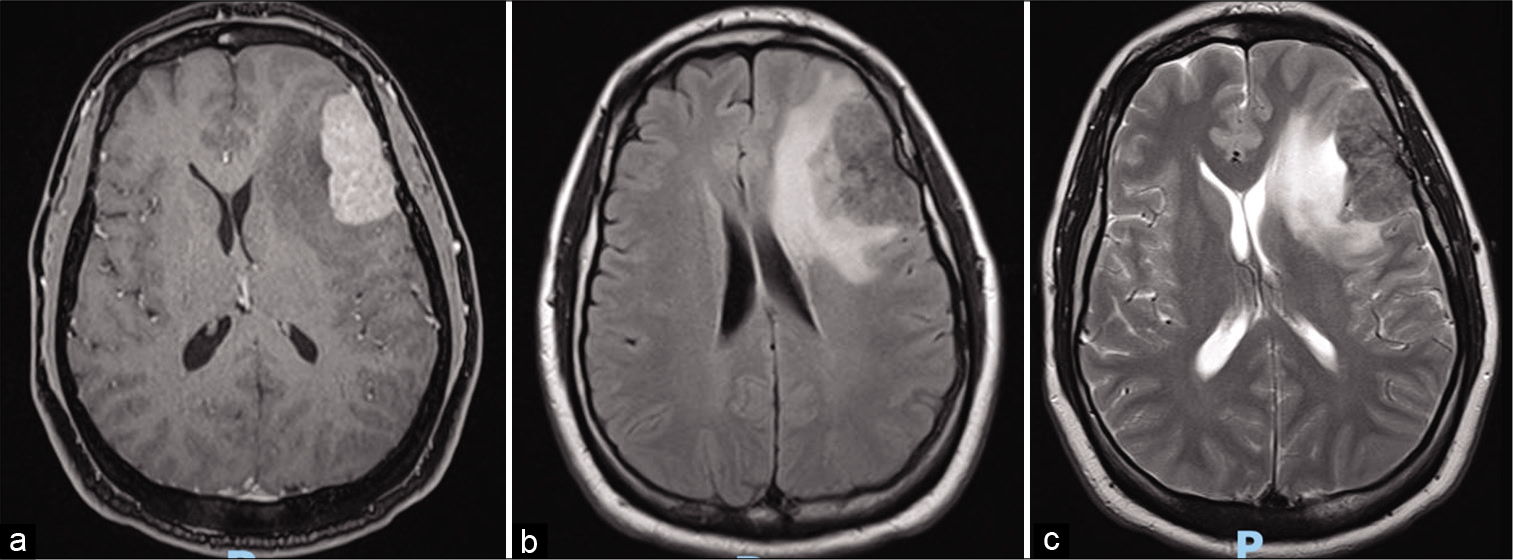
Male patient, 37 years old, presented 4 years before with painless left supraclavicular adenomegaly, with progressive increase followed by intense pain in the left clavicle after physical activity. Imaging examinations demonstrated the presence of bone infiltration, supraclavicular and infraclavicular adenomegaly, as well as lesions in the orbit and cranial cap. Biopsy of supraclavicular lymph node confirmed lymphadenitis with massive sinus histiocytosis compatible with RDD, with immunohistochemical examination demonstrating CD68 and S100 positive and CD30 and CD1a negative. The patient initially presented an excellent response with corticoids using, noting significant regression of adenomegaly, and general improvement of symptoms. In the last year, however, he began to refer to migratory arthralgia with an increase in cervical adenomegalies, requiring the continuous use of corticoids and increased doses in exacerbations, and he presented with pulsatile headache which was often disabling. A skull MRI was performed which revealed an expansive lesion in the left frontal region [
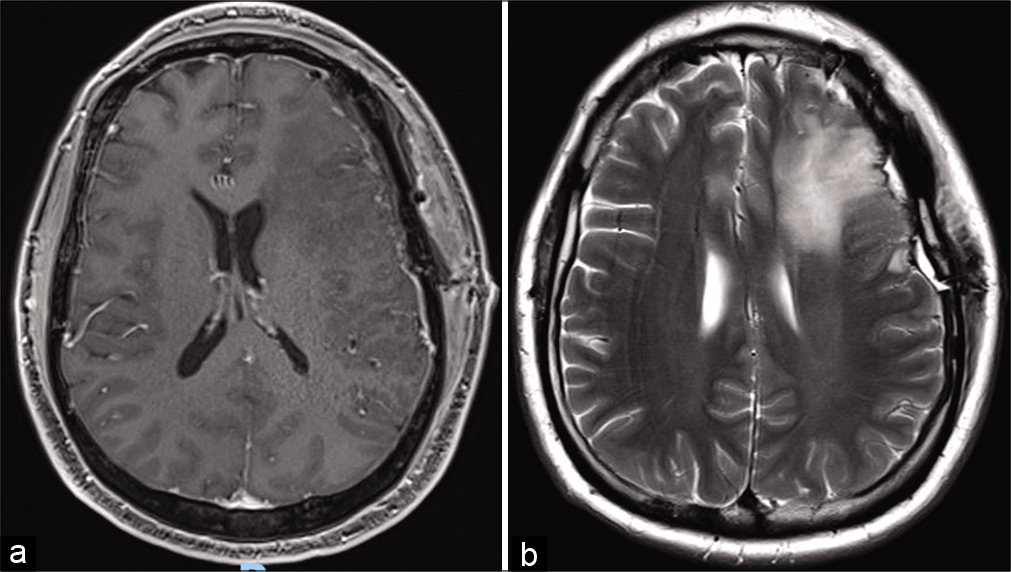
Figure 2:
Magnetic resonance imaging at the post operated showed total resection of the left frontal tumor associated with edema, determining deletion of the local cortical grooves, compression of the frontal horn of the left lateral ventricle and contralateral midline deviation. (a) Axial gadolinium-enhanced image. (b) Axial T2-weighted.
Second case report
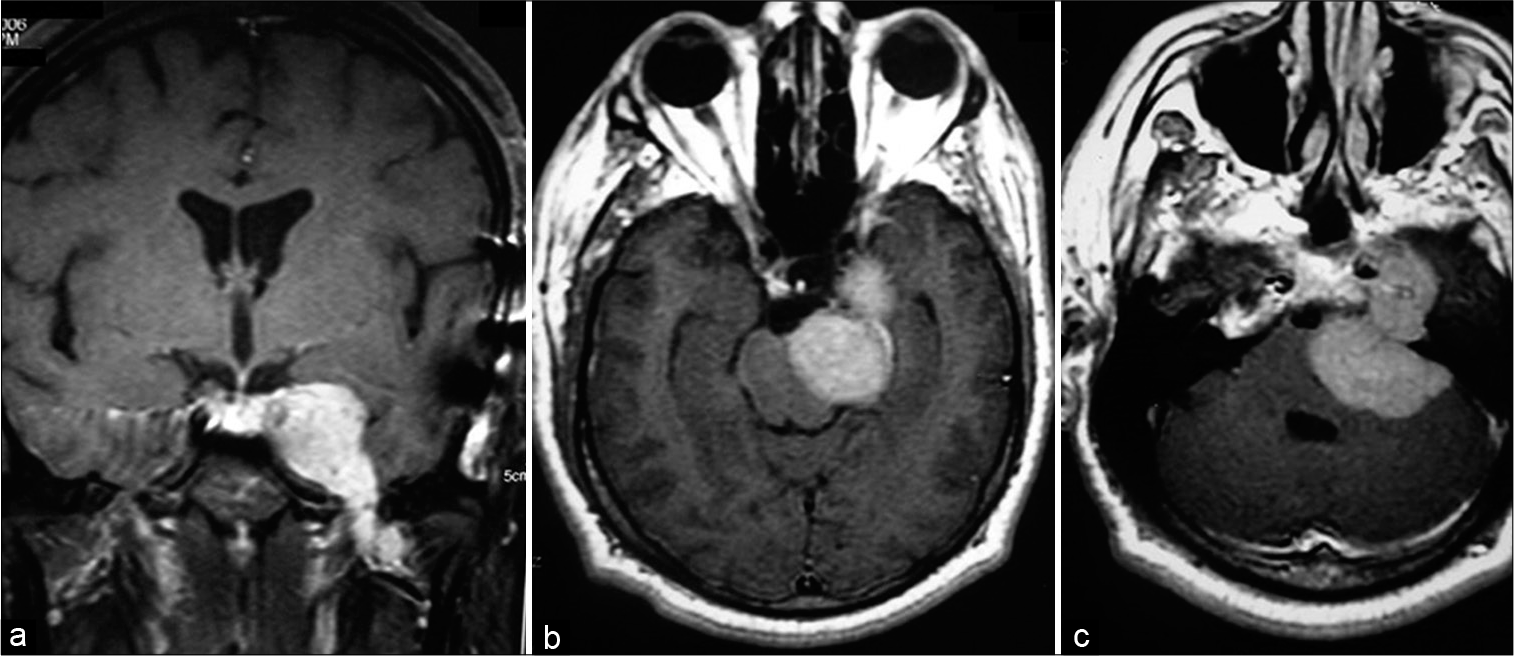
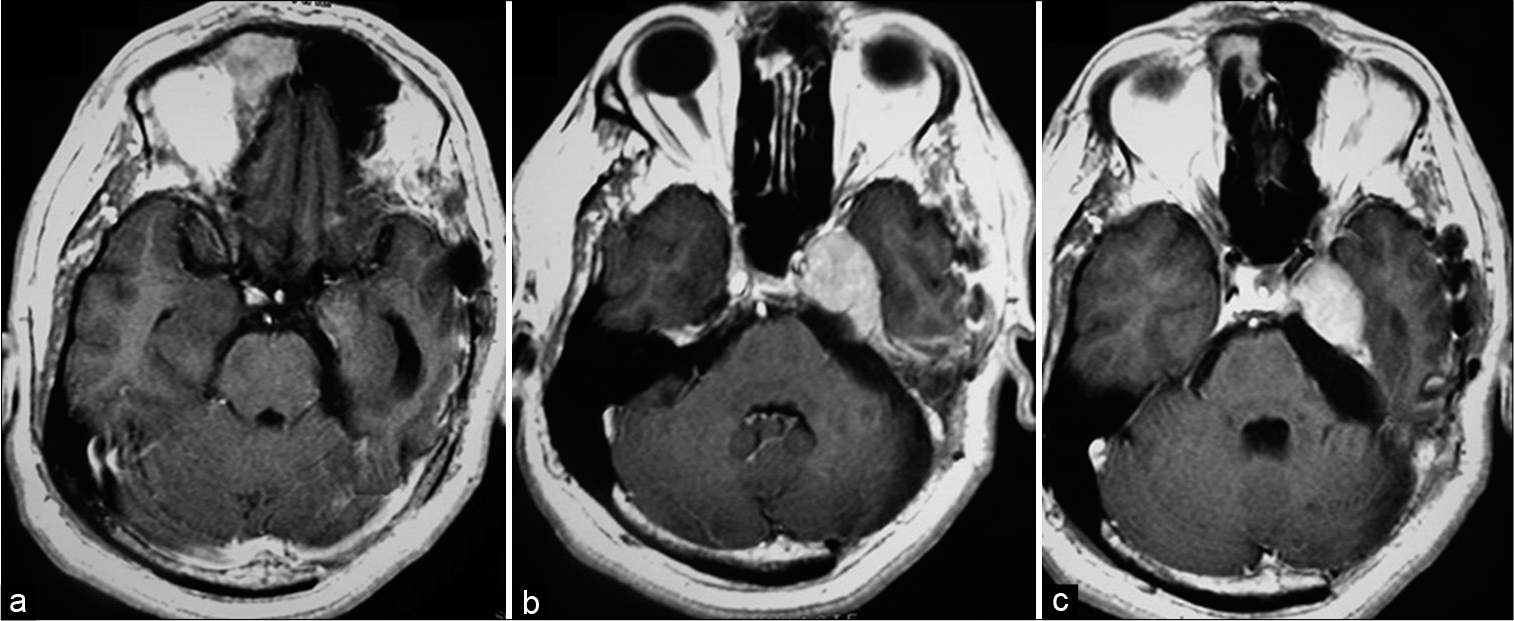
Male patient, 45 years old, receives specialized neurological care with convergent strabismus and complaint of diplopia, headache, ringing in the left ear and hypoacusis for 6 months. A gadolinium-contrasted MRI examination was requested, which demonstrated a lesion in the petroclival region invading the cavernous sinus with extension into the posterior fossa, with contrast uptake compatible with meningioma. MRI also showed that the lesion reached the cervical region, descending through the petroclival portion, and bordering the clivus [
Figure 3:
Magnetic resonance imaging preoperated showing expansive lesion in the left petroclival region with left to right mass effect, invading the cavernous sinus with extension into the posterior fossa and reach the cervical region, descending through the petroclival portion, bordering the clivus. Lesion with contrast enhances compatible with meningioma. (a) Coronal gadolinium-enhanced image (GEI). (b and c) Axial GEI.
DISCUSSION
According to 285 RDD literature reviewed cases [
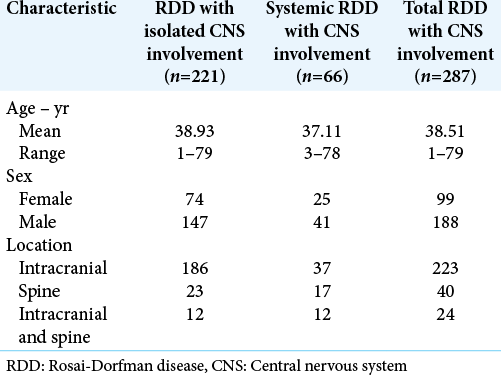
Table 1:
Characteristics of CNS-RDD cases according to present and previous reports from references.[
Table 2:
Central nervous system involvement in 287 cases of Rosai-Dorfman disease.[
On the other hand, comparing the frequencies of intracranial or spinal involvement according to the systemic or non-systemic involvement of the disease, RDD with isolated CNS involvement, reported in 77% (n = 221) of all cases of CNS-RDD, showed that 84% (n = 186) of isolated cases of the CNS had exclusively intracranial involvement and only 10% (n = 23) had exclusively spinal involvement. As for systemics CNS-RDD cases, the exclusively intracranial involvement occurred in 56% (n = 37) of the cases, while the exclusively spinal involvement was present in 26% (n = 17) of them. Therefore, it should be noted that comparing RDD with isolated CNS involvement, systemic CNS-RDD has a lower prevalence of exclusively intracranial involvement and a greater involvement of the spinal cord; in 26% (n = 17) of the cases with systemic presentation there was exclusive involvement of the spinal and in approximately 18% (n = 12) of the cases there was intracranial and spinal involvement. Thus, it is interesting to note that when RDD has systemic involvement, spinal cord involvement is more frequent than in relation to RDD with exclusive CNS involvement, which may have different explanations, such as perhaps because of systemic disease focus origin, usually sinus and with massive lymphadenopathy in the region, be closer to the spinal cord, this will somehow facilitate the disease spread to this nearest neural structure. This would mean that, once systemic RDD was present, it could spread more easily to any location in the CNS, without maintaining the preferential intracranial involvement of CNS exclusive cases.
The typical radiological findings of intracranial RDD show dural-based, extra-axial, well-circumscribed masses mimicking meningioma with MRI usually reveals multiple well-defined, dural-based or intraventricular, extra-axial masses with possible perilesional cerebral edema.[
As for the two reported cases of RDD, they were very similar to the expected age group and sex grouping, according to the literature and our review. As for the location of the lesion, which can happen in many regions, including the supratentorial region, where meningiomas occur and in which one of them mimics, the two cases presented in this study are very representative, especially the second one, since at first moment it was thought that it was one of those. Furthermore, the involvement reaching the cervical portion of the second case is compatible with a higher prevalence location of spinal cord injuries according to previous studies.[
More importantly, surgical resection should follow the same pattern as meningiomas, since the texture of both is very similar, and it is extremely unlikely that with only radiological images the two pathologies can be differentiated before neurosurgical removal for anatomopathological analysis. At present, the best treatment for intracranial RDD involves surgical excision,[
CONCLUSION
Thus, we conclude that intracranial Rosai-Dorfman tumors should always be remembered as differential diagnosis of meningiomas, since they are similar radiologically and macroscopically. Once remembered and diagnosed, the lesion must be treated following the same pattern of resection done in meningiomas and, treatment’s differences will not occur in the surgical excision technique, but in complementary chemotherapy implementation, radiotherapy, and even with radiosurgery aid, depending on the case. Thus, it is possible to obtain better results than with just the isolated surgical procedure.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abdel-Razek M, Matter GA, Azab WA, Katchy KC, Mallik AA. Isolated intracranial Rosai-Dorfman disease: Report of two cases and a review of the literature. Turk Neurosurg. 2013. 23: 509-13
2. Adeleye AO, Amir G, Fraifeld S, Shoshan Y, Umansky F, Spektor S. Diagnosis and management of Rosai-Dorfman disease involving the central nervous system. Neurol Res. 2010. 32: 572-8
3. Agnoletto GJ, Bit-Ivan EN, Hanel RA, Sauvageau E. All that glitters is not gold: Rosai-Dorfman as a single cerebellar necrotic lesion. BMJ Case Rep. 2019. 12: e228483
4. Alimli AG, Oztunali C, Boyunaga OL, Pamukcuoglu S, Okur A, Borcek AO. MRI and CT findings of isolated intracranial RosaiDorfman disease in a child. Neuroradiol J. 2016. 29: 146-9
5. Amagasa M, Yuda F, Kojima H, Noshita N, Sato S. Natural course of lymphocytic infundibuloneurohypophysitis. Clin Neuropathol. 2001. 20: 229-32
6. Ambati S, Chamyan G, Restrepo R, Escalon E, Fort J, Pefkarou A. Rosai-Dorfman disease following bone marrow transplantation for pre-B cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008. 51: 433-5
7. Andriko JA, Morrison A, Colegial CH, Davis BJ, Jones RV. Rosai-Dorfman disease isolated to the central nervous system: A report of 11 cases. Mod Pathol. 2001. 14: 172-8
8. Aouba A, Terrier B, Vasiliu V, Candon S, Brousse N, Varet B. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: Towards a new therapeutic approach. Haematologica. 2006. 91: ECR52-2
9. Arnao V, Riolo M, Savettieri G, Aridon P. Mercaptopurine treatment in an adult man with orbital and intracranial RosaiDorfman disease. Case Rep Neurol Med. 2016. 2016: 1030478
10. Asai A, Matsutani M, Kohno T, Fujimaki T, Tanaka H, Kawaguchi K. Leptomeningeal and orbital benign lymphophagocytic histiocytosis. Case report. J Neurosurg. 1988. 69: 610-2
11. Ayukawa F, Shibuya H, Yoshimura R, Watanabe H, Miura M. Curative brachytherapy for recurrent/residual tongue cancer. Strahlenther Onkol. 2007. 183: 133-7
12. Baassiri W, Moussalem CK, Massaad E, Zeidan YH, Darwish H. Craniocervical Rosai-Dorfman disease involving the vertebral artery: Case report and literature review. World Neurosurg. 2020. 133: 69-73
13. Battal B, Hamcan S, Balyemez U, Akgun V. Choroid plexus involvement in Rosai-Dorfman disease. Neurol India. 2017. 65: 222-3
14. Bernard F, Sarran N, Serre I, Baldet P, Callamand P, Margueritte G. Sinus histiocytosis (Destombes-RosaiDorfman disease) revealed by paraplegia. Arch Pédiatr. 1999. 6: 173-7
15. Beros V, Houra K, Rotim K, Zivkovic DJ, Cupic H, Kosec A. Isolated cerebellar intraparenchymal Rosai-Dorfman disease--case report and review of literature. Br J Neurosurg. 2011. 25: 292-6
16. Bertero L, Zenga F, Maletta F, Senetta R, Cassoni P. A 68-year-old woman with a left orbital and temporal mass. Brain Pathol. 2018. 28: 133-4
17. Bhandari A, Patel PR, Patel MP. Extranodal Rosai-Dorfman disease with multiple spinal lesions: A rare presentation. Surg Neurol. 2006. 65: 308-11
18. Bhat A, Kupanur SS, Geethamani V. Isolated intracranial rosaidorfman disease involving the meninges: Report of a rare case. Turk Neurosurg. 2015. 25: 186-9
19. Bhattacharjee MB, Wroe SJ, Harding BN, Powell M. Sinus histiocytosis with massive lymphadenopathy--isolated suprasellar involvement. J Neurol Neurosurg Psychiatry. 1992. 55: 156-8
20. Bing F, Brion JP, Grand S, Pasquier B, Lebas JF. Tumor arising in the periventricular region. Neuropathology. 2009. 29: 101-3
21. Boissaud-Cooke MA, Bhatt K, Hilton DA, Muquit S. Isolated intracranial Rosai-Dorfman disease: Case report and review of the literature. World Neurosurg. 2020. 137: 239-42
22. Brandsma D, Jansen GH, Spliet W, Van Nielen K, Taphoorn MJ. The diagnostic difficulties of meningeal and intracerebral plasma cell granulomas--presentation of three cases. J Neurol. 2003. 250: 1302-6
23. Breiner A, Dubinski W, Gray B, Munoz DG. A 63 year old woman with white matter lesions and pachymeningeal inflammation. Brain Pathol. 2013. 23: 225-8
24. Buchino JJ, Byrd RP, Kmetz DR. Disseminated sinus histiocytosis with massive lymphadenopathy: Its pathologic aspects. Arch Pathol Lab Med. 1982. 106: 13-6
25. Burger PC, Scheithauer BW.editors. Atlas of tumor pathology. Tumors of the Central Nervous System. Washington, DC: Armed Forces Institute of Pathology; 1994. p. 259-86
26. Camp SJ, Roncaroli F, Apostolopoulos V, Weatherall M, Lim S, Nandi D. Intracerebral multifocal Rosai-Dorfman disease. J Clin Neurosci. 2012. 19: 1308-10
27. Cao XY, Luan SH, Bao WM, Shen C, Yang BJ. Solitary intracranial Rosai-Dorfman disease: Case report and literature review. J Int Med Res. 2011. 39: 2045-50
28. Carey MP, Case CP. Sinus histiocytosis with massive lymphadenopathy presenting as a meningioma. Neuropathol Appl Neurobiol. 1987. 13: 391-8
29. Castellano-Sanchez AA, Brat DJ. May 2003: 57-year-old-woman with acute loss of strength in her right upper extremity and slurred speech. Brain Pathol. 2003. 13: 641-2
30. Catalucci A, Lanni G, Ventura L, Ricci A, Galzio RJ, Gallucci M. A rare case of intracranial rosai-dorfman disease mimicking multiple meningiomas. A case report and review of the literature. Neuroradiol J. 2012. 25: 569-74
31. Cavuoto K, Galor A, Dubovy SR, Gregori N, McCarthy M. Subconjunctival masses associated with central nervous system rosai-dorfman disease. Cornea. 2011. 30: 237-40
32. Chan KW, Chow YY, Ghadially FN, Stansfeld AG, Woo CH. Rosai-Dorfman disease presenting as spinal tumor. A case report with ultrastructural and immunohistochemical studies. J Bone Joint Surg Am. 1985. 67: 1427-31
33. Chen KT. Crush cytology of Rosai-Dorfman disease of the central nervous system. A report of 2 cases. Acta Cytol. 2003. 47: 1111-5
34. Chivukula S, Clark K, Murdoch G, Engh J. A singular case of intracranial sinus histiocytosis without massive lymphadenopathy: Isolated Rosai-Dorfman disease of the hypothalamus. J Neurol Surg Part A Cent Eur Neurosurg. 2014. 76: 244-8
35. Clark WC, Berry AD. Extranodal sinus histiocytosis with massive lymphadenopathy: Isolated central nervous system involvement mimicking meningioma. South Med J. 1996. 89: 621-3
36. Cooper SL, Jenrette JM. Rosai-Dorfman disease: Management of CNS and systemic involvement. Clin Adv Hematol Oncol. 2012. 10: 199-202
37. Cunliffe CH, Fischer I, Monoky D, Law M, Revercomb C, Elrich S. Intracranial lesions mimicking neoplasms. Arch Pathol Lab Med. 2009. 133: 101-23
38. Deodhare SS, Ang LC, Bilbao JM. Isolated intracranial involvement in Rosai-Dorfman disease: A report of two cases and review of the literature. Arch Pathol Lab Med. 1998. 122: 161-5
39. Deshayes E, Le Berre JP, Jouanneau E, Vasiljevic A, Raverot G, Seve P. 18F-FDG PET/CT findings in a patient with isolated intracranial Rosai-Dorfman disease. Clin Nucl Med. 2013. 38: e50-52
40. Destombes P. Adenitis with lipid excess, in children or young adults, seen in the Antilles and in Mali(4 cases). Bull Soc Pathol Exot Filiales. 1965. 58: 1169-75
41. Di Rocco F, Garnett MR, Puget S, Pueyerredon F, Roujeau T, Jaubert F. Cerebral localization of Rosai-Dorfman disease in a child. Case report. J Neurosurg. 2007. 107: 147-51
42. Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014. 124: 483-92
43. Diamond EL, Durham BH, Dogan A, Hyman DM, Rampal RK, Ulaner G. Phase 2 trial of single-agent cobimetinib for adults with BRAF V600-mutant and wild-type histiocytic disorders. Blood. 2017. 130: 257
44. Dran G, Rasendrarijao D, Vandenbos F, Paquis P. RosaiDorfman disease causing spinal cord compression. Neurosurgery. 2008. 62: E977-8
45. El Majdoub F, Brunn A, Berthold F, Sturm V, Maarouf M. Stereotactic interstitial radiosurgery for intracranial RosaiDorfman disease. A novel therapeutic approach. Strahlenther Onkol. 2009. 185: 109-12
46. El Molla M, Mahasneh T, Holmes SE, Al-Khawaja D. Rare presentation of Rosai-Dorfman disease mimicking a cervical intramedullary spinal cord tumor. World Neurosurg. 2014. 81: 442.e7-9
47. Forest F, N’guyen AT, Fesselet J, Metellus P, Bouvier C, de Paula AM. Meningeal Rosai-Dorfman disease mimicking meningioma. Ann Hematol. 2014. 93: 937-40
48. Fortea J, Compta Y, Valldeoriola F, Tolosa E, Rey MJ, Gastón F. Fatal worsening of late-onset cerebellar ataxia with neuronal intranuclear inclusions due to superimposed meningeal RosaiDorfman disease. Mov Disord. 2008. 23: 1488-90
49. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): Review of the entity. Semin Diagn Pathol. 1990. 7: 19-73
50. Foucar E, Rosai J, Dorfman RF, Brynes RK. The neurologic manifestations of sinus histiocytosis with massive lymphadenopathy. Neurology. 1982. 32: 365-72
51. Franco-Paredes C, Martin K. Extranodal Rosai-Dorfman disease involving the meninges. South Med J. 2002. 95: 1101-2
52. Friedman MJ, Rossoff LJ, Aftalion B, Khan A, Decker R, Steinberg H. Sinus histiocytosis presenting as a mediastinal mass. Chest. 1984. 86: 266-7
53. Fukushima T, Yachi K, Ogino A, Ohta T, Watanabe T, Yoshino A. Isolated intracranial Rosai-Dorfman disease without dural attachment--case report. Neurol Med Chir (Tokyo). 2011. 51: 136-40
54. Gaetani P, Tancioni F, Di Rocco M, Rodriguez y Baena R. Isolated cerebellar involvement in Rosai-Dorfman disease: Case report. Neurosurgery. 2000. 46: 479-81
55. Garces S, Medeiros LJ, Patel KP, Li S, Pina-Oviedo S, Li J. Mutually exclusive recurrent KRAS and MAP2K1 mutations in Rosai-Dorfman disease. Mod Pathol. 2017. 30: 1367-77
56. Geara AR, Ayoubi MA, Achram MC, Chamseddine NM. Rosai-Dorfman disease mimicking neurofibromatosis: Case presentation and review of the literature. Clin Radiol. 2004. 59: 625-30
57. Ghosal N, Murthy G, Visvanathan K, Sridhar M, Hegde AS. Isolated intracranial Rosai Dorfman disease masquerading as meningioma: A case report. Indian J Pathol Microbiol. 2007. 50: 382-4
58. Gies U, Gruia D, Lassmann H, Bergmann M. A case of rapidly progressive Rosai-Dorfman disease restricted to the central nervous system. Zentralbl Neurochir. 2005. 66: 142-6
59. Griffiths SJ, Tang W, Parameswaran R, Kelsey A, West CG. Isolated intracranial Rosai-Dorfman disease mimicking meningioma in a child. Br J Neurosurg. 2004. 18: 293-7
60. Gui Q, Li F, Song X. Intracranial Rosai-Dorfman disease: A report of seven cases with review of literature. Austin J Clin Pathol. 2014. 1: 1013
61. Gupta A, Farzal Z, Pandey A. An unusual cause of proptosis. BMJ Case Rep. 2015. 2015: bcr2015211741
62. Gupta DK, Suri A, Mahapatra AK, Mehta VS, Garg A, Sarkar C. Intracranial Rosai-Dorfman disease in a child mimicking bilateral giant petroclival meningiomas: A case report and review of literature. Child’s Nerv Syst. 2006. 22: 1194-200
63. Gupta K, Bagdi N, Sunitha P, Ghosal N. Isolated intracranial Rosai-Dorfman disease mimicking meningioma in a child: A case report and review of the literature. Br J Radiol. 2011. 84: e138-41
64. Haas RJ, Helmig MS, Prechtel K. Sinus histiocytosis with massive lymphadenopathy and paraparesis: Remission with chemotherapy. A case report. Cancer. 1978. 42: 77-80
65. Hadjipanayis CG, Bejjani G, Wiley C, Hasegawa T, Maddock M, Kondziolka D. Intracranial Rosai-Dorfman disease treated with microsurgical resection and stereotactic radiosurgery. Case report. J Neurosurg. 2003. 98: 165-8
66. Halelfadl S, Bougrine M, Fadli M, Elkettani F, Bellakhdar F. Rosai-Dorfman disease mimicking meningioma. Pan Arab J Neurosurg. 2007. 11: 89-94
67. Hargett C, Bassett T. Atypical presentation of sinus histiocytosis with massive lymphadenopathy as an epidural spinal cord tumor: A case presentation and literature review. J Spinal Disord Tech. 2005. 18: 193-6
68. Hashimoto K, Kariya S, Onoda T, Ooue T, Yamashita Y, Naka K. Rosai-Dorfman disease with extranodal involvement. Laryngoscope. 2014. 124: 701-4
69. Hassani J, Porubsky C, Berman C, Zager J, Messina J, Henderson-Jackson E. Intraperitoneal Rosai-Dorfman disease associated with clear cell sarcoma: First case report. Pathology. 2016. 48: 742-4
70. Hinduja A, Aguilar LG, Steineke T, Nochlin D, Landolfi JC. Rosai-Dorfman disease manifesting as intracranial and intraorbital lesion. J Neurooncol. 2009. 92: 117-20
71. Hingwala D, Neelima R, Kesavadas C, Thomas B, Kapilamoorthy TR, Radhakrishnan VV. Advanced MRI in Rosai-Dorfman disease: Correlation with histopathology. J Neuroradiol. 2011. 38: 113-7
72. Hollowell JP, Wolfla CE, Shah NC, Mark LP, Whittaker MH. Rosai-Dorman disease causing cervical myelopathy. Spine (Phila Pa 1976). 2000. 25: 1453-6
73. Hong Cheng SK, Tang YL, Prashanth RJ, Chuah KL. Extradural Brain Mass in a 64-Year-Old Man. Brain Pathol. 2017. 27: 115-6
74. Hong CS, Starke RM, Hays MA, Mandell JW, Schiff D, Asthagiri AR. Redefining the prevalence of dural involvement in Rosai-Dorfman disease of the central nervous system. World Neurosurg. 2016. 90: 702.e13-20
75. Huang BY, Zhang H, Zong WJ, Sun YH. Rosai-Dorfman disease of rare isolated spinal involvement: Report of 4 cases and literature review. World Neurosurg. 2016. 85: 367.e11-6
76. Huang HY, Huang CC, Lui CC, Chen HJ, Chen WJ. Isolated intracranial Rosai-Dorfman disease: Case report and literature review. Pathol Int. 1998. 48: 396-402
77. Idir I, Cuvinciuc V, Uro-Coste E, Penna M, Boetto S, Cognard C. MR perfusion of intracranial Rosai-Dorfman disease mimicking meningioma. J Neuroradiol. 2011. 38: 133-4
78. Imada H, Sakatani T, Sawada M, Matsuura T, Fukushima N, Nakano I. A lethal intracranial Rosai-Dorfman disease of the brainstem diagnosed at autopsy. Pathol Int. 2015. 65: 549-53
79. Jayaram A, Al Maslamani NJ, Rahiman NAPA, Negi VC. RosaiDorfman disease with paravertebral and epidural thoracic spine involvement: A case report and literature review. Radiol Case Rep. 2020. 15: 484-8
80. Jiang Y, Jiang S. Intracranial meningeal Rosai-Dorfman disease mimicking multiple meningiomas: 3 Case reports and a literature review. World Neurosurg. 2018. 120: 382-90
81. Johnston JM, Limbrick DD, Ray WZ, Brown S, Shimony J, Park TS. Isolated cerebellar Rosai-Dorfman granuloma mimicking Lhermitte-Duclos disease: Case report. J Neurosurg Pediatr. 2009. 4: 118-20
82. Jones MP, Rueda-Pedraza ME. Extranodal sinus histiocytosis with massive lymphadenopathy presenting as an intramedullary spinal cord tumor: A case report. Am J Hematol. 1997. 54: 253-7
83. Joshi SS, Joshi S, Muzumdar G, Turel KE, Shah RM, Ammbulkar I. Cranio-spinal Rosai Dorfman disease: Case series and literature review. Br J Neurosurg. 2019. 33: 176-83
84. Joubert C, Dagain A, Faivre A, Nguyen AT, Fesselet J, FigarellaBranger D. Intracranial Rosai-Dorfman disease mimicking multiple meningiomas. Rev Med Brux. 2013. 34: 112-4
85. Jurić G, Jakić-Razumović J, Rotim K, Zarković K. Extranodal sinus histiocytosis (Rosai-Dorfman disease) of the brain parenchyma. Acta Neurochir (Wien). 2003. 145: 145-9
86. Kaminsky J, Koerbel A, Mittelbronn M, Beschorner R, Ernemann U, Tatagiba M. Rosai-Dorfman disease involving the cranial base, paranasal sinuses and spinal cord. Clin Neuropathol. 2005. 24: 194-200
87. Kattner KA, Stroink AR, Roth TC, Lee JM. Rosai-Dorfman disease mimicking parasagittal meningioma: Case presentation and review of literature. Surg Neurol. 2000. 53: 452-7
88. Katz DS, Poe LB, Corona RJJ. Sinus histiocytosis with massive lymphadenopathy: A case of simultaneous upper respiratory tract and CNS disease without lymphadenopathy. AJNR Am J Neuroradiol. 1993. 14: 219-22
89. Kayali H, Onguru O, Erdogan E, Sirin S, Timurkaynak E. Isolated intracranial Rosai-Dorfman disease mimicking meningioma. Clin Neuropathol. 2004. 23: 204-8
90. Kelly WF, Bradey N, Scoones D. Rosai-Dorfman disease presenting as a pituitary tumour. Clin Endocrinol (Oxf). 1999. 50: 133-7
91. Kessler E, Srulijes C, Toledo E, Shalit M. Sinus histiocytosis with massive lymphadenopathy and spinal epidural involvement: A case report and review of the literature. Cancer. 1976. 38: 1614-8
92. Kidd DP, Revesz T, Miller NR. Rosai-Dorfman disease presenting with widespread intracranial and spinal cord involvement. Neurology. 2006. 67: 1551-5
93. Kim GG, Friedel ME, Eloy JA, Jyung RW, Liu JK. Extensive multifocal Rosai-Dorfman disease involving the central nervous system and paranasal sinuses. Laryngoscope. 2011. 121: S234-4
94. Kim M, Provias J, Bernstein M. Rosai-Dorfman disease mimicking multiple meningioma: Case report. Neurosurgery. 1995. 36: 1185-7
95. Kitai R, Llena J, Hirano A, Ido K, Sato K, Kubota T. Meningeal Rosai-Dorfman disease: Report of three cases and literature review. Brain Tumor Pathol. 2001. 18: 49-54
96. Kitai R, Sato K, Kubota T, Kabuto M, Kawano H, Kobayashi H. Meningeal sinus histiocytosis mimicking lymphoplasmacyte-rich meningioma. Case report. J Neurosurg. 1996. 84: 1051-4
97. Konca C, Özkurt ZN, Deger M, Akı Z, Yağcı M. Extranodal multifocal Rosai-Dorfman disease: Response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009. 89: 58-62
98. Kong Z, Wang Y, Ma W, Cheng X. FDG PET/CT image for a Rosai-Dorfman disease with pituitary and bone involvement in a pediatric patient. Clin Nucl Med. 2019. 44: 873-5
99. Konishi E, Ibayashi N, Yamamoto S, Scheithauer BW. Isolated intracranial Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy). Am J Neuroradiol. 2003. 24: 515-8
100. Koot RW, Maarouf M, Hulshof MC, Voges J, Treuer H, Koedooder C. Brachytherapy: Results of two different therapy strategies for patients with primary glioblastoma multiforme. Cancer. 2000. 88: 2796-802
101. Kozak B, Talbott J, Uzelac A, Rehani B. Rosai-Dorfman disease isolated to the thoracic epidural spine. J Radiol Case Rep. 2015. 9: 6-16
102. Krishnamoorthy V, Parmar CF, Panikar D. Isolated intracranial Rosai Dorfman disease. Neurol India. 2011. 59: 443-6
103. Kumar KK, Menon G, Nair S, Radhakrishnan VV. RosaiDorfman disease mimicking chronic subdural hematoma. J Clin Neurosci. 2008. 15: 1293-5
104. Kumar YA, Peng PY, Chen XC. Intracranial rosai-dorfman disease. Case Rep Radiol. 2014. 2014: 724379
105. Kutlubay Z, Bairamov O, Sevim A, Demirkesen C, Mat MC. Rosai-Dorfman disease: A case report with nodal and cutaneous involvement and review of the literature. Am J Dermatopathol. 2014. 36: 353-7
106. Lauwers GY, Perez-Atayde A, Dorfman RF, Rosai J. The digestive system manifestations of Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy): Review of 11 cases. Hum Pathol. 2000. 31: 380-5
107. Le Guenno G, Galicier L, Uro-Coste E, Petitcolin V, Rieu V, Ruivard M. Successful treatment with azathioprine of relapsing Rosai-Dorfman disease of the central nervous system. J Neurosurg. 2012. 117: 486-9
108. Leung JL, Cheung JY, Tan TC, Tang KW, Chan CM, Ho LC. Carotid artery occlusion in a patient with intracranial RosaiDorfman disease. J Hong Kong Coll Radiol. 2003. 6: 211-3
109. Li Y, Sun H, Zhang Y, Liu W. Isolated intracranial RosaiDorfman disease presenting as mental deterioration. Clin Neurol Neurosurg. 2012. 114: 1070-3
110. Lima LB, Sobreira-Neto MA, Braga-Neto P, Nóbrega PR. Isolated central nervous system Rosai-Dorfman disease and breast cancer: An unusual presentation. Int J Neurosci. 2019. 129: 393-6
111. Löhr HF, Gödderz W, Wölfe T, Heike M, Knuth A, Meyer zum Büschenfelde KH. Long-term survival in a patient with Rosai-Dorfman disease treated with interferon-alpha. Eur J Cancer. 1995. 31A: 2427-8
112. Long E, Lassalle S, Cheikh-Rouhou R, Hofman V, Lacour JP, Hofman P. Intestinal occlusion caused by Rosai-Dorfman disease mimicking colonic diverticulitis. Pathol Res Pract. 2007. 203: 233-7
113. Lopez P, Estes ML. Immunohistochemical characterization of the histiocytes in sinus histiocytosis with massive lymphadenopathy: Analysis of an extranodal case. Hum Pathol. 1989. 20: 711-5
114. Lou X, Chen Z, Wang F, Ma L. MR findings of RosaiDorfman disease in sellar and suprasellar region. Eur J Radiol. 2012. 81: 1231-7
115. Lu CH, Chang KC, Lee EJ, Chuang MT, Chang RS. Intracranial Rosai-Dorfman disease with unusual transcranial extension. J Neuroimaging. 2012. 22: 312-5
116. Lu D, Estalilla OC, Manning JT, Medeiros LJ. Sinus histiocytosis with massive lymphadenopathy and malignant lymphoma involving the same lymph node: A report of four cases and review of the literature. Mod Pathol. 2000. 13: 414-9
117. Lu M, Guo DY. Leptomeningeal Rosai-Dorfman disease. J Neuroradiol. 2010. 37: 196-7
118. Lüdemann W, Banan R, Samii A, Koutzoglou M, Di Rocco C. Cerebral Rosai-Dorfman disease. Childs Nerv Syst. 2015. 31: 529-32
119. Lungren MP, Petrella JR, Cummings TJ, Grant GA. Isolated intracranial Rosai-Dorfman disease in a child. Am J Neuroradiol. 2009. 30: E148-9
120. Luo Z, Zhang Y, Zhao P, Lu H, Yang K, Zhang Y. Characteristics of Rosai-Dorfman disease primarily involved in the central nervous system: 3 Case reports and review of literature. World Neurosurg. 2017. 97: 58-63
121. Lutterbach J, Henne K, Pagenstecher A, Böhm J. Lung cancer and Rosai-Dorfman’s disease. A clinicopathological study. Strahlenther Onkol. 2003. 179: 486-92
122. Mahzoni P, Zavareh MH, Bagheri M, Hani N, Moqtader B. Intracranial Rosai-Dorfman disease. J Res Med Sci. 2012. 17: 304-7
123. Maiti TK, Gangadharan J, Mahadevan A, Arivazhagan A, Chandramouli BA, Shankar SK. Rosai-Dorfman disease presenting as cervical extradural lesion: A case report with review of literature. Neurol India. 2011. 59: 438-42
124. Maklad AM, Bayoumi Y, Tunio M, Alshakweer W, Dahar MA, Akbar SA. Steroid-resistant extranodal rosai-dorfman disease of cheek mass and ptosis treated with radiation therapy. Case Rep Hematol. 2013. 2013: 428297
125. Maric I, Pittaluga S, Dale JK, Niemela JE, Delsol G, Diment J. Histologic features of sinus histiocytosis with massive lymphadenopathy in patients with autoimmune lymphoproliferative syndrome. Am J Surg Pathol. 2005. 29: 903-11
126. McPherson CM, Brown J, Kim AW, Demonte F. Regression of intracranial Rosai-Dorfman disease following corticosteroid therapy: Case report. J Neurosurg. 2006. 104: 840-4
127. Miletic H, Röhling R, Stenzel W, Deckert M, Benz-Bohm G, Berthold F. 8-year-old child with a lesion in the left nucleus lentiformis. Brain Pathol. 2008. 18: 598-601
128. Mir R, Aftalion B, Kahn LB. Sinus histiocytosis with massive lymphadenopathy and unusual extranodal manifestations. Arch Pathol Lab Med. 1985. 109: 867-70
129. Mirra SS, Tindall SC, Check IJ, Brynes RK, Moore WW. Inflammatory meningeal masses of unexplained origin. An ultrastructural and immunological study. J Neuropathol Exp Neurol. 1983. 42: 453-68
130. Morandi X, Godey B, Riffaud L, Heresbach N, Brassier G. Isolated Rosai-Dorfman disease of the fourth ventricle. Case illustration. J Neurosurg. 2000. 92: 890
131. Morgan N V, Morris MR, Cangul H, Gleeson D, StraatmanIwanowska A, Davies N. Mutations in SLC29A3, encoding an equilibrative nucleoside transporter ENT3, cause a familial histiocytosis syndrome (Faisalabad histiocytosis) and familial Rosai-Dorfman disease. PLoS Genet. 2010. 6: e1000833
132. Nalini A, Jitender S, Anantaram G, Santosh V. Rosai Dorfman disease: Case with extensive dural involvement and cerebrospinal fluid pleocytosis. J Neurol Sci. 2012. 314: 152-4
133. Nassif S, Boulos F. Extranodal (dural) Rosai-Dorfman disease radiologically and histologically mimicking meningioma: A case report. Anal Quant Cytopathol Histopathol. 2015. 37: 144-6
134. Natarajan S, Post KD, Strauchen J, Morgello S. Primary intracerebral rosai-dorfman disease: A case report. J Neurooncol. 2000. 47: 73-7
135. Ng HK, Poon WS. Sinus histiocytosis with massive lymphadenopathy localized to the sella. Br J Neurosurg. 1995. 9: 551-5
136. O’Malley DP, Duong A, Barry TS, Chen S, Hibbard MK, Ferry JA. Co-occurrence of Langerhans cell histiocytosis and Rosai-Dorfman disease: Possible relationship of two histiocytic disorders in rare cases. Mod Pathol. 2010. 23: 1616-23
137. Olsen EA, Crawford JR, Vollmer RT. Sinus histiocytosis with massive lymphadenopathy. Case report and review of a multisystemic disease with cutaneous infiltrates. J Am Acad Dermatol. 1988. 18: 1322-32
138. Osenbach RK. Isolated extranodal sinus histiocytosis presenting as an intramedullary spinal cord tumor with paraplegia. Case report. J Neurosurg. 1996. 85: 692-6
139. Panicker NK, Sabhikhi AK, Rai R. Rosai-Dorfman disease presenting as a meningioma. Indian J Cancer. 1996. 33: 192-4
140. Parmar V, Seward C, Huho A, Qian J, Gandhi R, Pilitsis JG. Rosai-Dorfman disease presenting as cervical radiculopathy. Clin Neurol Neurosurg. 2013. 115: 808-10
141. Patwardhan PP, Goel NA. Isolated intraventricular RosaiDorfman disease. Asian J Neurosurg. 2018. 13: 1285-7
142. Petzold A, Thom M, Powell M, Plant GT. Relapsing intracranial Rosai-Dorfman disease. J Neurol Neurosurg Psychiatry. 2001. 71: 538-41
143. Pinkawa M, Fischedick K, Piroth MD, Gagel B, Borchers H, Jakse G. Health-related quality of life after permanent interstitial brachytherapy for prostate cancer: Correlation with postimplant CT scan parameters. Strahlenther Onkol. 2006. 182: 660-5
144. Prayson RA, Rowe JJ. Dural-based Rosai-Dorfman disease: Differential diagnostic considerations. J Clin Neurosci. 2014. 21: 1872-3
145. Pulsoni A, Anghel G, Falcucci P, Matera R, Pescarmona E, Ribersani M. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): Report of a case and literature review. Am J Hematol. 2002. 69: 67-71
146. Purav P, Ganapathy K, Mallikarjuna VS, Annapurneswari S, Kalyanaraman S, Reginald J. Rosai-Dorfman disease of the central nervous system. J Clin Neurosci. 2005. 12: 656-9
147. Qin G, Ye J, Lan S, Liang Y, Xu P, Tang X. Rosai-Dorfman disease with spinal and multiple intracranial involvement: A case report and literature review. Br J Neurosurg. 2019. 18: 1-5
148. Ramos AA, Vega MA, Alles JV, Garcia MJ, Martínez AM. Multiple involvement of the central nervous system in RosaiDorfman disease. Pediatr Neurol. 2012. 46: 54-6
149. Raslan O, Ketonen LM, Fuller GN, Schellingerhout D. Intracranial Rosai-Dorfman disease with relapsing spinal lesions. J Clin Oncol. 2008. 26: 3087-9
150. Raslan OA, Schellingerhout D, Fuller GN, Ketonen LM. Rosai-Dorfman disease in neuroradiology: Imaging findings in a series of 10 patients. AJR Am J Roentgenol. 2011. 196: W187-93
151. Resnick DK, Johnson BL, Lovely TJ. Rosai-Dorfman disease presenting with multiple orbital and intracranial masses. Acta Neuropathol. 1996. 91: 554-7
152. Richardson TE, Wachsmann M, Oliver D, Abedin Z, Ye D, Burns DK. BRAF mutation leading to central nervous system rosai-dorfman disease. Ann Neurol. 2018. 84: 147-52
153. Rivera D, Pérez-Castillo M, Fernández B, Stoeter P. Long-term follow-up in two cases of intracranial Rosai-Dorfman Disease complicated by incomplete resection and recurrence. Surg Neurol Int. 2014. 5: 30
154. Rocha-Maguey J, Felix-Torrontegui JA, Cabrera-López M, Gutiérrez-Castro M, Montante-Montes de Oca D. A new case of cervical intramedullary sinus histiocytosis causing paraplegia and review of the literature. Surg Neurol Int. 2016. 7: 9
155. Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol. 1969. 87: 63-70
156. Rosai J.editors. Rosai and Ackerman’s Surgical Pathology. New York: Elsevier Health Sciences; 2011. p.
157. Rotondo F, Munoz DG, Hegele RG, Gray B, Khatun N, Bonert M. Rosai-Dorfman disease involving the neurohypophysis. Pituitary. 2010. 13: 256-9
158. Russo N, Giangaspero F, Beccaglia MR, Santoro A. Intracranial dural histiocytosis. Br J Neurosurg. 2009. 23: 449-54
159. Said R, Abi-Fadel F, Talwar J, Attallah JP, Dilawari A. Intracranial rosai-dorfman: A clinical challenge. Neurologist. 2011. 17: 117-9
160. Sakai K, Koike G, Seguchi K, Nakazato Y. Sinus histiocytosis with massive lymphadenopathy: A case of multiple dural involvement. Brain Tumor Pathol. 1998. 15: 63-9
161. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, Velazquez-Vega J, Borja MJ, Rosenberg S. Rosai-Dorfman disease of the central nervous system: Report of 6 cases and review of the literature. Medicine (Baltimore). 2014. 93: 165-75
162. Sasaki K, Pemmaraju N, Westin JR, Wang WL, Khoury JD, Podoloff DA. A single case of rosai-dorfman disease marked by pathologic fractures, kidney failure, and liver cirrhosis treated with single-agent cladribine. Front Oncol. 2014. 4: 297
163. Sato A, Sakurada K, Sonoda Y, Saito S, Kayama T, Jokura H. Rosai-Dorfman disease presenting with multiple intracranial and intraspinal masses: A case report. No Shinkei Geka. 2003. 31: 1199-204
164. Schmidt S, Eich G, Hanquinet S, Tschäppeler H, Waibel P, Gudinchet F. Extra-osseous involvement of Langerhans’ cell histiocytosis in children. Pediatr Radiol. 2004. 34: 313-21
165. Scumpia AJ, Frederic J-A, Cohen AJ, Bania M, Hameed A, Xiao PQ. Isolated intracranial Rosai-Dorfman disease with orbital extension. J Clin Neurosci. 2009. 16: 1108-9
166. Seyednejad F, Tubbs RS, Shoja MM, Daghigi MH, Oakes WJ. Presumed recurrence of intracranial Rosai-Dorfman disease as a cervical spine tumor. Acta Neurochir (Wien). 2007. 149: 425-7
167. Shah V, Mohyeldin A, London NR, Fritz J, Prevedello DM, Carrau RL. When a meningioma isn’t: Endoscopic endonasal orbital decompression and biopsy of skull base Rosai-Dorfman disease treated previously with empiric radiation therapy. World Neurosurg. 2020. 135: 141-5
168. Shaver EG, Rebsamen SL, Yachnis AT, Sutton LN. Isolated extranodal intracranial sinus histiocytosis in a 5-year-old boy. Case report. J Neurosurg. 1993. 79: 769-73
169. Shuangshoti SS, Navalitloha Y, Sukpanichnant S, Unhasuta C, Shuangshoti S. Central nervous system involvement in Rosai-Dorfman disease: Report of a case with a review of the literature. Neuropathology. 1999. 19: 341-6
170. Siadati A, Powell SZ, Shahab I, Valadka AB, Parker JR. Pathologic quiz case: A 48 year-old woman with a dural-based intracranial tumor. Arch Pathol Lab Med. 2001. 125: 1115-6
171. Simos M, Dimitrios P, Philip T. A new clinical entity mimicking meningioma diagnosed pathologically as rosai-dorfman disease. Skull Base Surg. 1998. 8: 87-92
172. Siu RCH, Tan IL, Davidson AS, Robertson A, Fraser CL. Clinical Reasoning: Compressive optic neuropathy secondary to intracranial Rosai-Dorfman disease. Neurology. 2015. 85: e89-92
173. Song SK, Schwartz IS, Strauchen JA, Huang YP, Sachdev V, Daftary DR. Meningeal nodules with features of extranodal sinus histiocytosis with massive lymphadenopathy. Am J Surg Pathol. 1989. 13: 406-12
174. Sundaram C, Uppin SG, Prasad BC, Sahu BP, Devi MU, Prasad VS. Isolated Rosai Dorfman disease of the central nervous system presenting as dural-based and intraparenchymal lesions. Clin Neuropathol. 2005. 24: 112-7
175. Symss NP, Cugati G, Vasudevan MC, Ramamurthi R, Pande A. Intracranial Rosai Dorfman disease: Report of three cases and literature review. Asian J Neurosurg. 2010. 5: 19-30
176. Tamrazi B, Shiroishi MS, Liu CS. Advanced imaging of intracranial meningiomas. Neurosurg Clin. 2016. 27: 137-43
177. Tan S, Ruan L, Jin K, Wang F, Mou J, Huang H. Systemic Rosai-Dorfman disease with central nervous system involvement. Int J Neurosci. 2018. 128: 192-7
178. Tanboon J, Chaipipat M, Wattanasirmkit V, Wongtabtim W, Shuangshoti S, Bunyaratavej K. Squash cytology of RosaiDorfman disease in the sellar region. Acta Cytol. 2003. 47: 1143-4
179. Tasso M, Esquembre C, Blanco E, Moscardo C, Niveiro M, Paya A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2 chlorodeoxyadenosine. Pediatr Blood Cancer. 2006. 47: 612-5
180. Taufiq M, Khair A, Begum F, Akhter S, Shamim Farooq M, Kamal M. Isolated intracranial Rosai-Dorfman disease. Case Rep Neurol Med. 2016. 2016: 1972594
181. Tauziede-Espariat A, Polivka M, Chabriat H, Bouazza S, Sene D, Adle-Biassette H. A case report of meningeal RosaiDorfman disease associated with IgG4-related disease. Clin Neuropathol. 2015. 34: 343-9
182. Tavangar SM, Mahta A, Haghpanah V, Larijani B. Extranodal Rosai-Dorfman disease involving the meninges in a 79-year-old man. Ann Saudi Med. 2006. 26: 474-6
183. Theeler BJ, Keylock JB, Yoest SM. Teaching NeuroImage: Isolated intracranial Rosai-Dorfman disease mimicking a meningioma. Neurology. 2008. 70: e42
184. Tian Y, Wang J, Ge J, zhao Ma Z, Ge M. Intracranial RosaiDorfman disease mimicking multiple meningiomas in a child: A case report and review of the literature. Child’s Nerv Syst. 2015. 31: 317-23
185. Tian Y, Wang J, Li M, Lin S, Wang G, Wu Z. RosaiDorfman disease involving the central nervous system: Seven cases from one institute. Acta Neurochir (Wien). 2015. 157: 1565-71
186. Toguri D, Louie A V, Rizkalla K, Franklin J, Rodrigues G, Venkatesan V. Radiotherapy for steroid-resistant laryngeal Rosai-Dorfman disease. Curr Oncol. 2011. 18: e158-62
187. Toh CH, Chen YL, Wong HF, Wei KC, Ng SH, Wan YL. RosaiDorfman disease with dural sinus invasion. Report of two cases. J Neurosurg. 2005. 102: 550-4
188. Tomio R, Katayama M, Takenaka N, Imanishi T. Complications of surgical treatment of Rosai-Dorfman disease: A case report and review. Surg Neurol Int. 2012. 3: 1
189. Triana-Pérez AB, Sánchez-Medina Y, Rosario PD, MillánCorada AM, Gómez-Perals LF, Domínguez-Báez JJ. Enfermedad de Rosai-Dorfman intracraneal: Presentación de un caso y revisión de la literatura. Neurocirugia. 2011. 22: 255-60
190. Tripathi R, Serajee F, Jiang H, Huq AH. Novel presentation of Rosai-Dorfman histiocytosis with a prolonged course of cranial and peripheral neuropathies. Pediatr Neurol. 2017. 71: 70-2
191. Trudel M. Dural involvement in sinus histiocytosis with massive lymphadenopathy. Case report. J Neurosurg. 1984. 60: 850-2
192. Tselis N, Kolotas C, Birn G, Röddiger S, Filipowicz I, Kontova M. CT-guided interstitial HDR brachytherapy for recurrent glioblastoma multiforme. Long-term results. Strahlenther Onkol. 2007. 183: 563-70
193. Tu J, Li WT, Yang C. Rosai-Dorfman disease of the subdural spine with a long segment lesion: A case report and literature review. J Int Med Res. 2017. 45: 875-81
194. Tubbs RS, Kelly DR, Mroczek-Musulman EC, Hammers YA, Berkow RL, Oakes WJ. Spinal cord compression as a result of Rosai-Dorfman disease of the upper cervical spine in a child. Childs Nerv Syst. 2005. 21: 951-4
195. Türe U, Seker A, Bozkurt SU, Uneri C, Sav A, Pamir MN. Giant intracranial Rosai-Dorfman disease. J Clin Neurosci. 2004. 11: 563-6
196. Udono H, Fukuyama K, Okamoto H, Tabuchi K. RosaiDorfman disease presenting multiple intracranial lesions with unique findings on magnetic resonance imaging. Case report. J Neurosurg. 1999. 91: 335-9
197. Vaiselbuh SR, Bryceson YT, Allen CE, Whitlock JA, Abla O. Updates on histiocytic disorders. Pediatr Blood Cancer. 2014. 61: 1329-35
198. Varan A, Şen H, Akalan N, Oğuz KK, Sağlam A, Akyüz C. Pontine Rosai-Dorfman disease in a child. Childs Nerv Syst. 2015. 31: 971-5
199. Varrassi M, Corridore A, Tommasino E, Giorgia S, Bruno F, Di Sibio A. MR imaging of cerebral involvement of RosaiDorfman disease: A single-centre experience with review of the literature. Radiol Med. 2021. 126: 89-98
200. Walker RN, Nickles TP, Lountzis NI, Jacobs DL, Nawaz NK. Rosai-Dorfman disease with massive intracranial involvement: Asymmetric response to conservative therapy. J Neuroimaging. 2011. 21: 194-6
201. Wang C, Kuang P, Xu F, Hu L. Intracranial Rosai-Dorfman disease with the petroclival and parasellar involvement mimicking multiple meningiomas: A case report and review of literature. Medicine (Baltimore). 2019. 98: e15548
202. Wang E, Anzai Y, Paulino A, Wong J. Rosai-Dorfman disease presenting with isolated bilateral orbital masses: report of two cases. AJNR Am J Neuroradiol. 2001. 22: 1386-8
203. Wang Y, Gao X, Tang W, Jiang C. Rosai-Dorfman disease isolated to the central nervous system: A report of six cases. Neuropathology. 2010. 30: 154-8
204. Warrier R, Chauhan A, Jewan Y, Bansal S, Craver R. RosaiDorfman disease with central nervous system involvement. Clin Adv Hematol Oncol. 2012. 10: 196-8
205. Wen JH, Wang C, Jin YY, Xu D, Jiang B, He XJ. Radiological and clinical findings of isolated meningeal RosaiDorfman disease of the central nervous system. Medicine (Baltimore). 2019. 98: e15365
206. Woodcock RJ, Mandell JW, Lipper MH. Sinus histiocytosis (Rosai-Dorfman disease) of the suprasellar region: MR imaging findings--a case report. Radiology. 1999. 213: 808-10
207. Wrzolek MA, Zagzag D. May 2002: 38-year-old man and 69-year-old woman with dural based masses. Brain Pathol. 2002. 12: 517-8
208. Wu L, Xu Y. Rosai-Dorfman disease: A rare lesion with dura tail sign mimicking spinal meningioma. Spine J. 2014. 14: 3058-9
209. Wu M, Anderson AE, Kahn LB. A report of intracranial RosaiDorfman disease with literature review. Ann Diagn Pathol. 2001. 5: 96-102
210. XiaoWen D, XueBin X, YuQing Y, Ting L. Intracranial RosaiDorfman disease: Case report and literature review. Eur J Radiol Extra. 2010. 76: e75-8
211. Xie Y, Pittaluga S, Price S, Raffeld M, Hahn J, Jaffe ES. Bone marrow findings in autoimmune lymphoproliferative syndrome with germline FAS mutation. Haematologica. 2017. 102: 364-72
212. Yang X, Liu J, Ren Y, Richard SA, Zhang Y. Isolated intracranial Rosai-Dorfman disease mimicking petroclival meningioma in a child. Medicine (Baltimore). 2017. 96: e8754
213. Yao K, Li TF, Zhu MW, Duan ZJ, Hu ZL, Bian Y. An intramedullary cervical cord lesion in a 12-year-old girl. Neuropathology. 2013. 33: 582-5
214. Yetiser S, Cekin E, Tosun F, Yildirim A. Rosai-Dorfman disease associated with neurosensorial hearing loss in two siblings. Int J Pediatr Otorhinolaryngol. 2004. 68: 1095-100
215. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006. 202: 165-70
216. Zhang H, Rödiger LA, Shen T, Miao J, Oudkerk M. Perfusion MR imaging for differentiation of benign and malignant meningiomas. Neuroradiology. 2008. 50: 525-30
217. Zhang JT, Tian HJ, Lang SY, Wang XQ. Primary intracerebral Rosai-Dorfman disease. J Clin Neurosci. 2010. 17: 1286-8
218. Zhang S, Huang J, Chen Y. Primary isolated intracranial RosaiDorfman disease: Report of a rare case and review of the literature. Neurol Neurochir Pol. 2018. 52: 390-3
219. Zhang TT, Fu YJ, Piao YS, Liu GZ, Wang LM, Chen SY. 43 year old woman with left arm paralysis. Brain Pathol. 2018. 28: 1021-2
220. Zhu F, Zhang J, Xing X, Wang D, Zhu R, Zhang Q. RosaiDorfman disease: A retrospective analysis of 13 cases. Am J Med Sci. 2013. 345: 200-10
221. Zhu H, Qiu LH, Dou YF, Wu JS, Zhong P, Jiang CC. Imaging characteristics of Rosai-Dorfman disease in the central nervous system. Eur J Radiol. 2012. 81: 1265-72
222. Ziemlewski A, Zienkiewicz J, Serkies K, Badzio A. Preliminary report of pulsed dose rate brachytherapy in head-and-neck cancer. Strahlenther Onkol. 2007. 183: 512-6