- Department of Medical Imaging, Northern Ontario School of Medicine, Sudbury,
- Department of Clinical Neurological Sciences, University of Western Ontario, London, Canada.
- Department of Medical Imaging, University of Western Ontario, London, Canada.
Correspondence Address:
Maksim Son, Department of Clinical Neurological Sciences, University of Western Ontario, London, Canada.
DOI:10.25259/SNI_69_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ruba Kiwan1, Maksim Son2, Michael Mayich3, Melfort Boulton2, Sachin Pandey3, Manas Sharma3. Ruptured intracranial infectious aneurysms: Single Canadian center experience. 06-May-2022;13:185
How to cite this URL: Ruba Kiwan1, Maksim Son2, Michael Mayich3, Melfort Boulton2, Sachin Pandey3, Manas Sharma3. Ruptured intracranial infectious aneurysms: Single Canadian center experience. 06-May-2022;13:185. Available from: https://surgicalneurologyint.com/surgicalint-articles/11586/
Abstract
Background: Ruptured intracranial infected aneurysms (IIAs) are relatively rare, but they portend high mortality. To the best of our knowledge, there is no Canadian case series on IIA, as well there is a relative paucity of international published experiences. Our purpose is to share the experience of a single Canadian tertiary center in managing ruptured IIA and to conduct a systematic review.
Methods: We did a retrospective case review series of adult patients with ruptured IIA treated at our institution. Second, we conducted a systematic review of the literature on ruptured IIA between 2011 and 2021 inclusive.
Results: At our institution, of a total eight cases with ruptured IIA, four were treated endovascularly and two by surgical bypass. For the systematic review, we included nine noncomparative studies with a total of 509 patients (318 males) and at least 437 ruptured IIA aneurysms. Favorable outcome was specified for 63.3% of patients (n = 57). Regarding ruptured IIA, favorable clinical outcome was described in 59.3% (n = 16).
Conclusion: This study highlights a single Canadian tertiary center experience in the management of IIA and compares it to the global trends of the past 10 years in a systematic review.
Keywords: Aneurysm, Endovascular, Infectious, Intracranial, Mycotic, Ruptured
INTRODUCTION
Intracranial infected aneurysms (IIAs) are a relatively rare complication of systemic infection. Due to their rarity, most of the available evidence is limited to retrospective single-center studies and case reports. We summarize evidence from the literature of the past decade and share our experience related to unique IIA features, as well as management and prognosis of ruptured IIAs.
IIAs are a complication of an ongoing systemic infection causing microbic infiltration and degradation of arterial vessel wall.[
Despite the paucity of evidence, endovascular treatment of IIA is an attractive option. Unlike noninfectious aneurysms, IIAs tend to affect more distal intracranial vessels[
Even for those clinical scenarios for which open neurosurgical treatment is necessary, endovascular treatments and intraoperative digital subtraction angiograms (DSA) may serve as useful adjuncts.
MATERIALS AND METHODS
Our series
We performed a retrospective chart review over a 10-year period (2010–2020) of all cases with ruptured IIA who were treated at the London Health Sciences Centre. Relevant data included age, gender, initial neurological condition, underlying diagnosis, number, location and characteristic of aneurysm, treatment, isolated bacteria, and clinical/ radiological outcome.
All patients data were collected according to the Canadian Tri-Council policy statement on ethical conduct for research involving the secondary use of data originally collected for health-care purposes.
Literature search
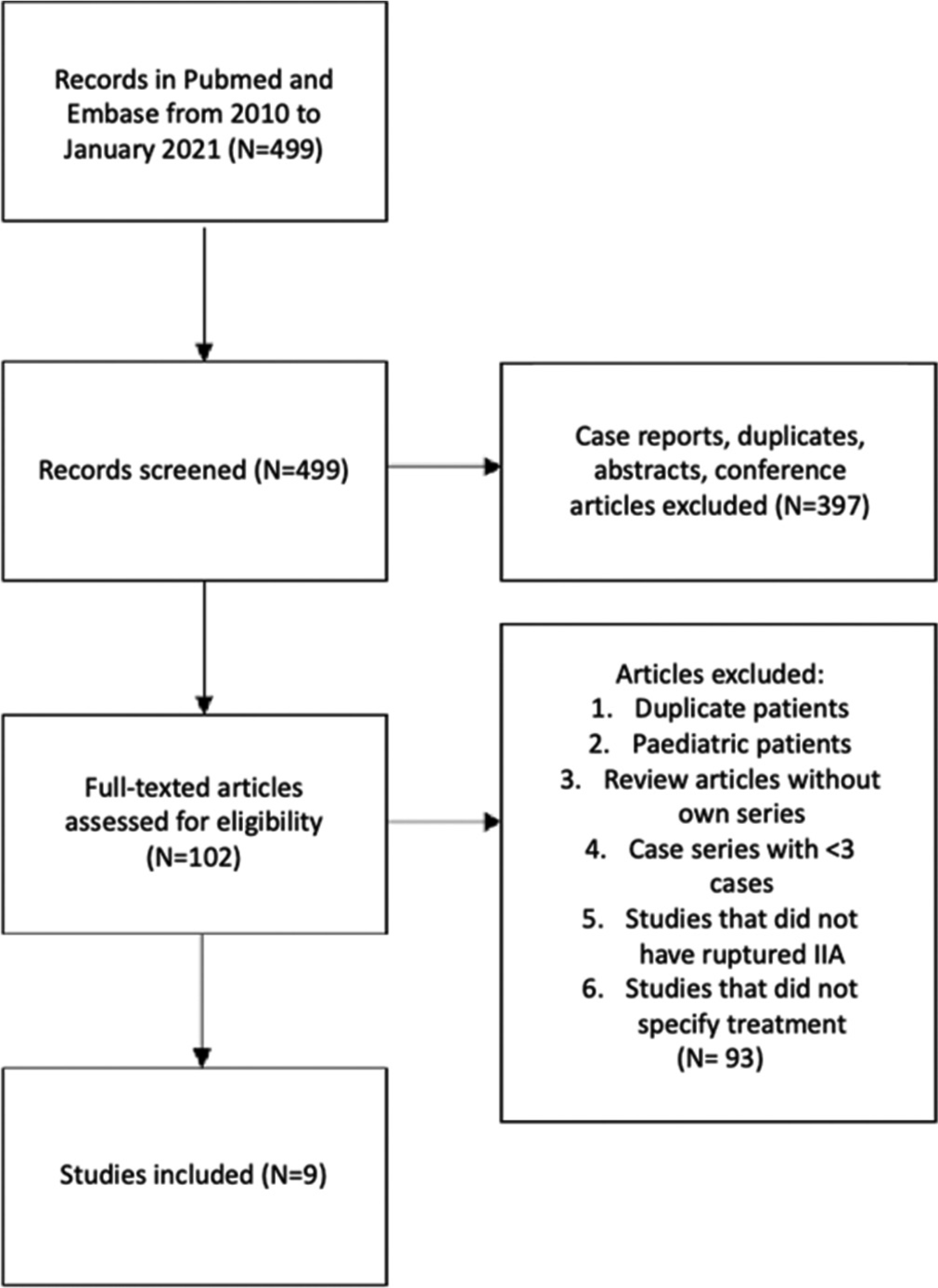
We performed a comprehensive literature search using PubMed and EMBASE databases using the following terms: “aneurysm* AND (cereb* OR brain OR *cranial*) AND (infect* OR mycotic OR bacterial).” Wild character (*) was used to include variations of the words and to improve the sensitivity of the search. We limited our search to English language publications between 2010 and January 2021. The flowchart of the literature review and exclusion criteria is summarized in [
Data included the number of patients in each study, age, gender, median number of aneurysms, number of ruptured aneurysm cases, mean/median aneurysm size and shape, aneurysm locations, treatment modality, and favorable outcome. For aneurysm location, we considered “distal” as any aneurysm at the level of M3, M4, P3, P4, A2, and artery of Heubner. For clinical outcome, we considered “favorable” outcome as modified Rankin scale (MRS) score of 2 or less or as a Glasgow Outcome Scale (GOS) score 4 and 5. In cases, where neither MRS nor GOS was available, we considered “favorable” outcome if the study used the terms “good recovery,” “no deficits,” “functional baseline,” “no morbidity,” and “not worsened.”
RESULTS
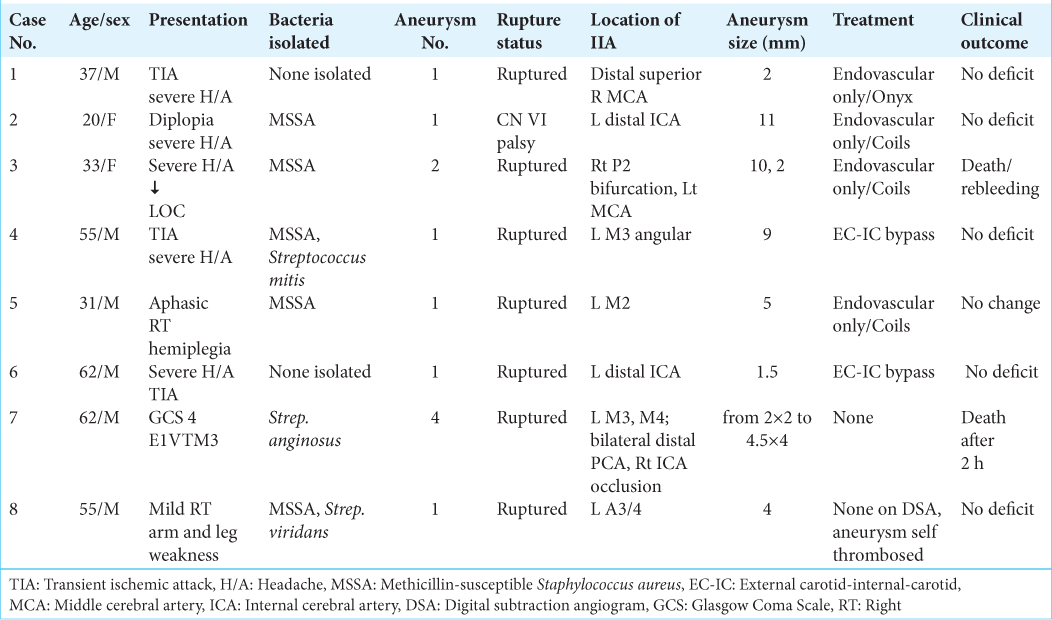
At our institution, eight patients were diagnosed with ruptured IIA: six men and two women with a median age of 46 years (range from 20 to 62 years). Six out of eight patients included in the case series presented within the past 5 years. Baseline demographics, clinical presentation, aneurysm characteristics, treatment options, and clinical outcomes are summarized in [
Case No.
Four cases were treated through endovascular therapy (three patients treated with coiling and one patient was treated using Onyx-18); two cases surgical extracranial-intracranial (EC-IC) bypass using intraoperative diagnostic angiogram and mapping of the arteries. In two cases, no treatment was performed as one patient died shortly (2 h) after the initial presentation with GCS 4 and CTA demonstrating multiple intracranial aneurysms (case number 7). In the other case, an intracranial aneurysm was seen in CTA, and on next day, DSA demonstrated spontaneous thrombosis (case number 8); on close follow-up, the aneurysm remained thrombosed.
Literature results
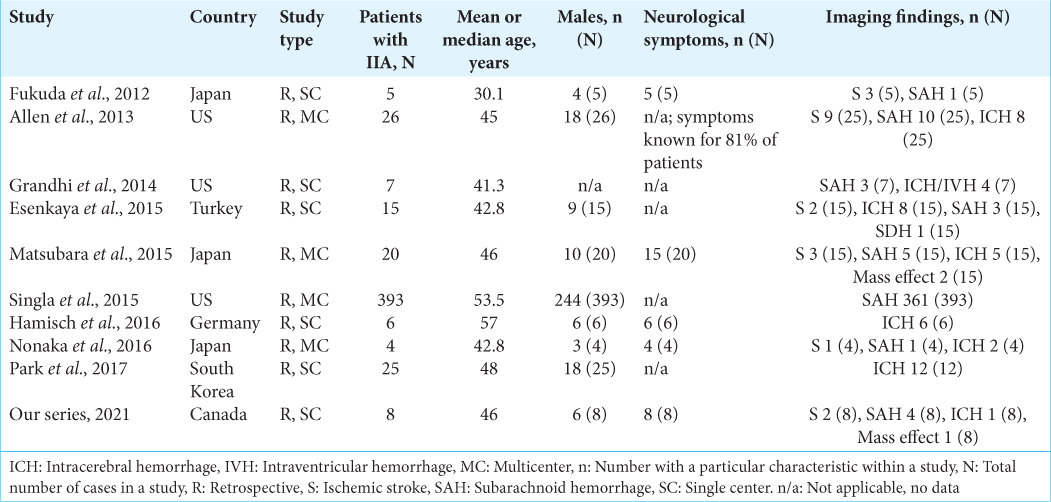
We included nine studies from the past 10 years into the final analysis. Without exception, all studies were retrospective chart reviews. Most studies were originated from a single center, with a few exceptions.[
Data from 509 patients with IIA were collected. Males composed 62.4% (n = 318) of all known cases. Detailed demographics and numbers of clinical symptoms are summarized in [
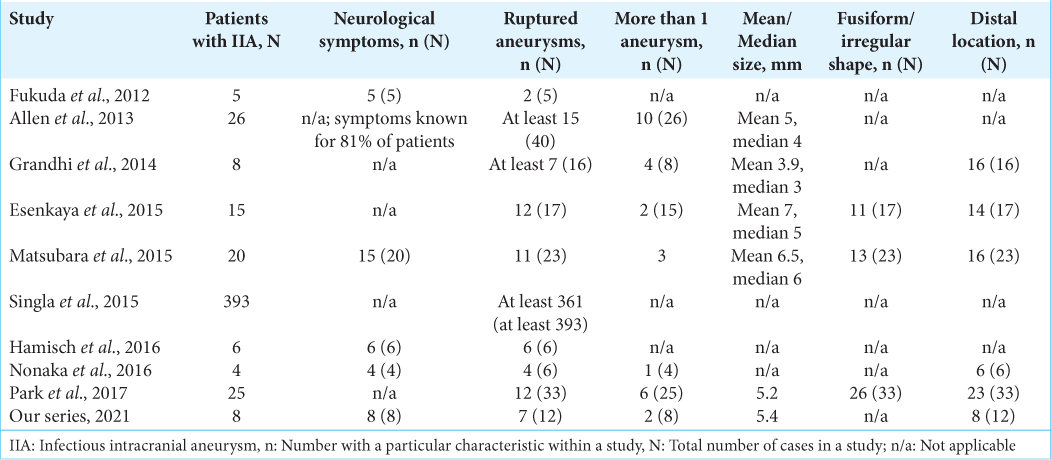
The percentage of ruptured IIA was at least 79.3% (at least 437 IIA out of at least 551 intracranial aneurysms). Only some studies reported aneurysm characteristics: 31.5% of patients had multiple IIA (28 out of 89 patients); 68.5% of IIA had irregular or fusiform shape (50 out of 73 IIA); and 77.6% of IIA were located distally (83 out of 107 IIA). Detailed aneurysm characteristics are found in [
Of the studies that reported on treatment modalities, more patients underwent endovascular treatment than open surgical treatment: while 22.7% of patients (n = 115) underwent endovascular treatment, only 11.4% of patients (n = 55) had open surgical treatment. It should be noted that the majority of patients in one large cohort have not received any intervention.[
In addition to our own case series, only six studies reported clinical outcomes.[
Illustrative cases from our center
Case 1. Endovascular embolization of ruptured IIA with Onyx 18
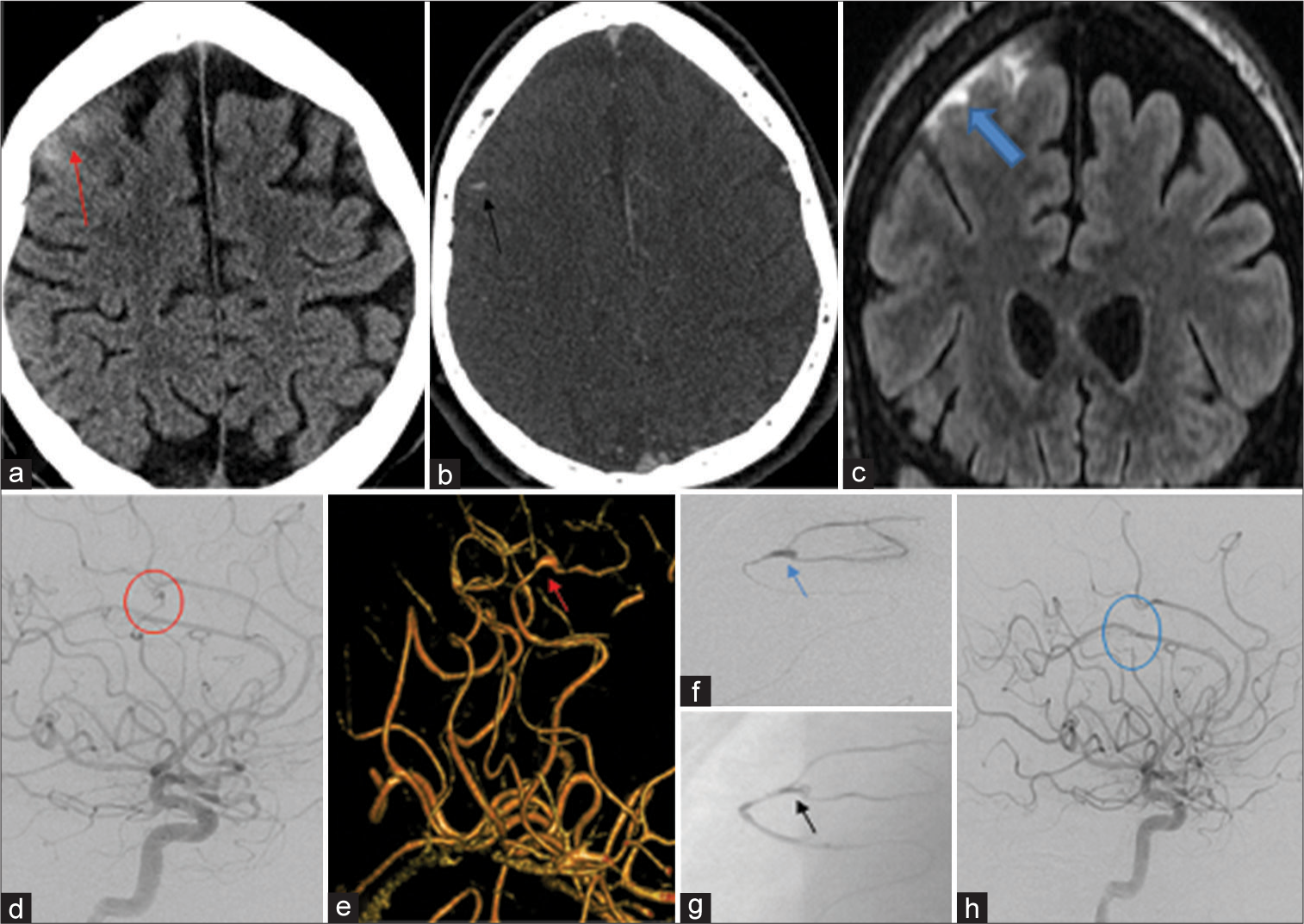
Patient 1 was a 37-year-old male with a remote history of intravenous drug use and IE resulting in severe aortic insufficiency, eventually requiring a porcine valve. He was admitted for aortic root abscess. While no bacteria were isolated, the presumed etiology was a dental infection. During his admission, 1 day before cardiac surgery, he developed severe headache with transient word finding difficulty. He was found to have focal subarachnoid hemorrhage (SAH) secondary to the right middle cerebral artery (MCA) IIA with a trace of the right frontal convexity SAH on computed tomography (CT) [
Figure 2:
A 36-year-old male admitted for aortic root abscess treatment and developed slurred speech. Noncontrast brain CT (a) demonstrated trace of subarachnoid hemorrhage over the right frontal convexity (red arrow), CTA (b) demonstrated tiny outpouching (black arrow) in a distal branch of the right middle cerebral artery in the region of the subarachnoid hemorrhage. Coronal FLAIR sequence MRI (c) confirmed the subarachnoid hemorrhage (blue arrow). Cerebral angiogram (DSA) right ICA injection lateral view (d) showed an irregular 2 mm aneurysm (red circle) in the distal branch of the superior division of the right middle cerebral artery. The small aneurysm was better seen on 3D image (e) (red arrow) and on the microcatheter injection (blue arrow) (f) Pre-embolization and postembolization (g) image with the onyx cast filling the aneurysm (black arrow) and the supplying arterial pedicle. Follow-up 6 months postembolization right ICA injection lateral view (h) demonstrated complete occlusion of the aneurysm (blue circle) with maintained patency of the MCA.
A decision was made in liaison with the cardiothoracic surgery team to treat the aneurysm before the cardiac surgery.
Procedure: under general anesthesia, a 6Fr 80 cm Shuttle sheath guide catheter (Cook Medical Inc., Bloomington, USA) was positioned in the proximal ICA, through which a 5 Fr Sofia intermediate catheter (MicroVention, Tustin, California) was positioned in the M1 segment of the right MCA, an Apollo microcatheter (Ev3, Irvine, CA) with a 1.5 cm detachable tip was navigated over a Balt Hybrid 008 (Balt, Montmorency, France) microwire into the deeper branch of the MCA. Angiography through the Apollo microcatheter confirmed the feeder of the mycotic aneurysm. The branch was embolized using Onyx 18 (Ev3, Irvine, CA) liquid embolic agent. There was complete occlusion of the inflow and outflow to the mycotic aneurysm. There was no evidence of contrast extravasation or nontarget vessel occlusion. The patient remained asymptomatic after the procedure, and next day, he underwent cardiac surgery for his aortic root abscess. Follow-up angiogram after 6 months revealed persistent complete occlusion of the aneurysm [
Case 2. Endovascular embolization of symptomatic IIA aneurysm with coils
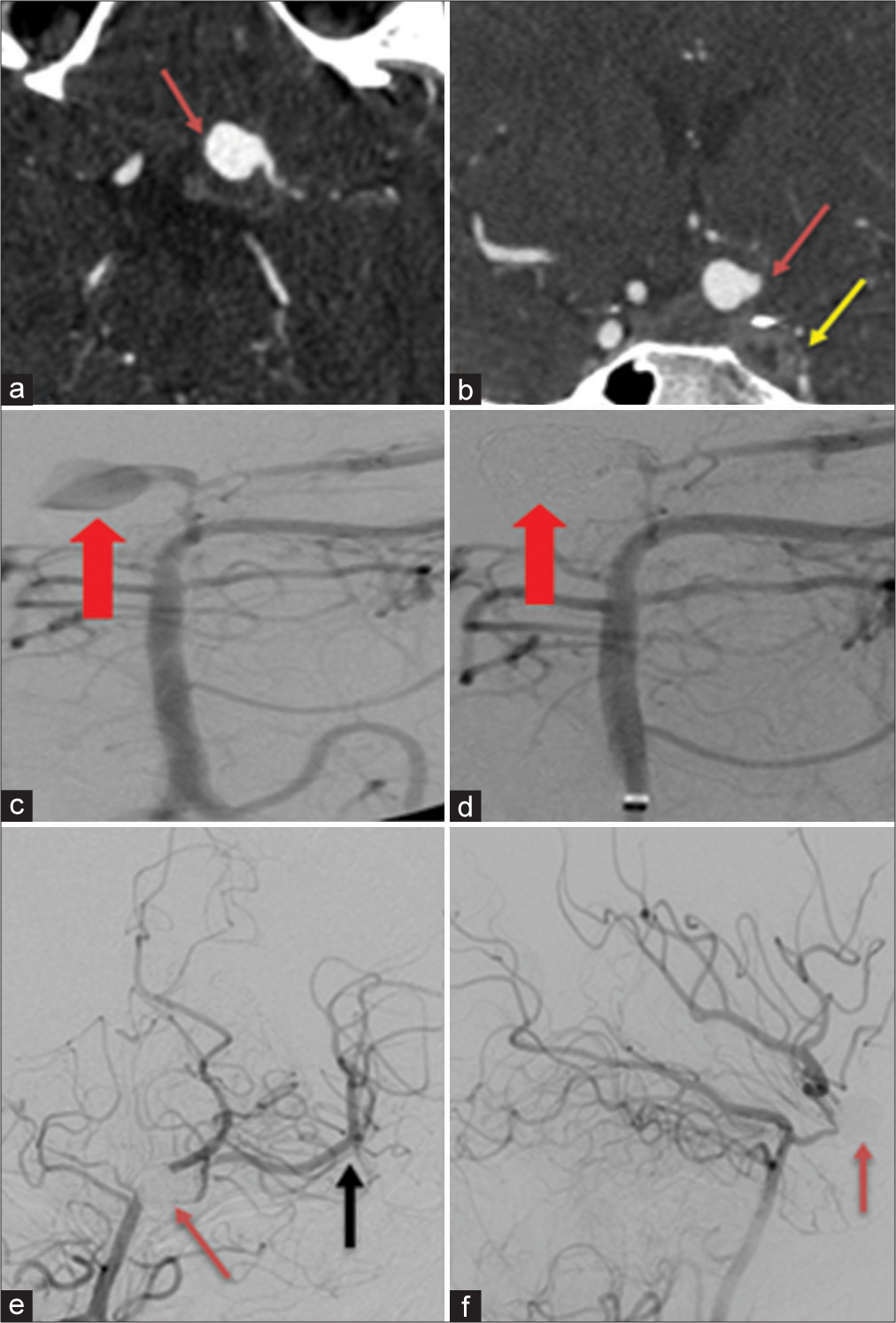
Patient 2 was a 19-year-old female with extensive medical history dating back to a motor vehicle accident at age of 10 years. She initially sustained a mesenteric vascular injury and there have been numerous confounding issues resulting in extensive medical therapy since. This has included MSSA bacteremia, osteomyelitis, and endocarditis. After a 2-week therapeutic period of IV antibiotics, she developed severe headache and diplopia. On presentation, she was found to have a sixth nerve palsy and CT/CTA [
Figure 3:
A 19-year-old female with the left cranial nerve VI palsy, CTA axial image (a) demonstrates saccular aneurysm from communicating segment of the left ICA (red arrow). Coronal plane (b) shows small lobulated extension anterolaterally (red arrow) and thrombosed left ICA with no contrast opacification (yellow arrow) compared to the contra lateral side which is normally opacified (not labeled). Cerebral angiogram right vertebral injection preembolization (c) demonstrates a 10 mm irregular lobulated aneurysm (red arrow) extending from the dysplastic communicating segment of left ICA. Post coil embolization (d), there is complete occlusion of the aneurysm (red arrow). One-month follow-up cerebral angiogram right vertebral injection anterior-posterior projection (e and f) shows the coil pack to be stable (red arrow) with complete occlusion and no evidence of recurrence. Persistent antegrade flow in the left middle cerebral artery (black arrow).
Cerebral angiogram demonstrated total ICA occlusion from the cervical bifurcation. The right vertebral injection [
Given the demonstration of robust collateral filling in the left MCA, PCOM sacrifice was left as a secondary treatment option should any increase in aneurysm filling or other worrisome change present on follow-up imaging.
The headache and sixth nerve palsy improved and she was discharged home after 2 days after a repeat single-vessel DSA confirmed stability of the coil pack with complete IIA occlusion (not shown).
On a 1-month follow-up, cerebral angiogram [
Case 3: Endovascular embolization of ruptured IIA with coils
A 33-year-old female had a complex medical history of Crohn’s disease, total colectomy and ileostomy, multiple cutaneous fistulae, and endocarditis related to a peripherally inserted central catheter (PICC) line infection.
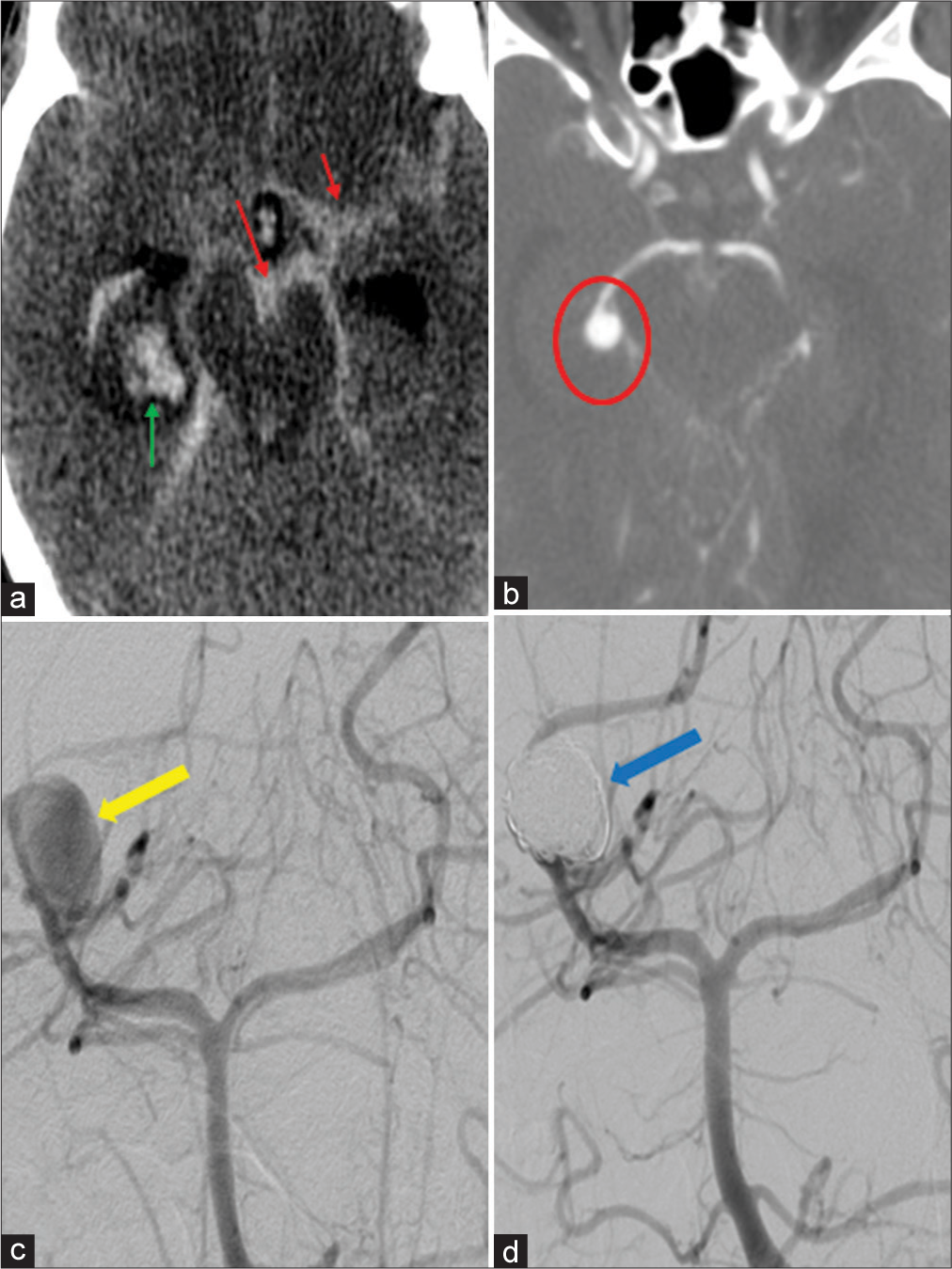
During her admission for the treatment of recurrent PICC line infection, mitral valve endocarditis, and pneumonia, she developed headache and decreased level of consciousness. CT head scan [
Figure 4:
A 33-year-old female with decreased level of consciousness; noncontrast CT (a) demonstrates diffuse SAH in the prepontine cistern and the right Sylvian fissure (red arrows), in addition to intraparenchymal hemorrhage in the right temporal lobe (green arrow). On CTA (b), a 9 mm round aneurysm extending from the sidewall of the right P2/3 junction (red circle) is seen. DSA AP view pre coil embolization (c) shows the aneurysm (yellow arrow) and postembolization (d) complete occlusion with no flow at the coil pack (blue arrow).
After multidisciplinary consideration, a decision was made to take an endovascular approach to the aneurysm and with a secondary option of parent artery sacrifice. DSA [
After the procedure, the patient awoke with no deficits. She was kept in an intensive care unit for close observation. After 6 h, her level of consciousness deteriorated and her GCS was found to be 3. Repeat CT demonstrated increased SAH and hydrocephalus. In light of these findings and her clinical status, her family elected for a palliative course to her care, and she passed shortly thereafter.
DISCUSSION
Aneurysm characteristics
IIA is rare; in one single-center retrospective study, IIA represented only 0.5% of all diagnosed intracranial aneurysms (33 IIA out of 7229 intracranial aneurysms).[
One study reports that while the absence of focal neurological deficits or even intracranial hemorrhage had both low sensitivity and specificity for the presence of IIA.[
Other differences between noninfectious aneurysms and IIA that may have impact treatment include aneurysm shape, location, and number of aneurysms: IIA tends to have an irregular or fusiform shape, is more distally located, and is more numerous than noninfectious aneurysms.[
Management of ruptured IIA
At present, there are no consensus guidelines for the management of ruptured IIA. Initial antimicrobial therapy remains a staple treatment strategy for all suspected IIA. The cases of symptomatic, large, rapidly expanding, or ruptured IIA are treated with urgent endovascular or open surgical intervention. In our series, 5 patients (62.5%) had excellent clinical outcomes with full resolution of deficits on follow-up, which supports the finding that 63.3% of patients (n = 57) had favorable outcomes [
Endovascular therapy options are diverse: liquid embolization (with Onyx or NCBI)[
In cases where open surgery is indicated, such as in the management of increased intracranial pressure, neurosurgeons have several options. Open surgical options include IIA clipping,[
Prognosis of IIA
Despite increased experience and a wide choice of interventional options, mortality remains high, at 10– 30% for patients with unruptured IIA and nearly 80% in patients with ruptured IIA.[
Limitations
The majority of studies included in this article are retrospective single-center case series, which are more likely to report positive results. As such, we were unable to control for publication bias. Furthermore, since the effect estimates could not be calculated, we could not directly assess the degree of publication bias. To partially mitigate this issue, we excluded studies and reports with <3 patients, thereby limiting our analysis to only nine studies. Another inherent methodological limitation of working with retrospective and noncomparative studies is the fact that direct comparisons cannot be made. Despite our best efforts to synthesize the evidence of the past decade, our systematic review cannot be used to draw generalizable conclusions.
CONCLUSION
In the past few years, there has been a trend for patients to be treated more often by endovascular approaches. However, in the absence of definitive data, the decision for the best treatment option for a given patient with a ruptured IIA should be taken after a multidisciplinary discussion of both open and endovascular neurovascular physicians.
This study highlights a single Canadian tertiary center experience in the management of ruptured IIA and compares it to the global trends of the past 10 years in a systematic review.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alawieh A, Chaudry MI, Turner RD, Turk AS, Spiotta AM. Infectious intracranial aneurysms: A systematic review of epidemiology, management, and outcomes. J Neurointerv Surg. 2018. 10: 708-16
2. Allen LM, Fowler AM, Walker C, Derdeyn CP, Nguyen BV, Hasso AN. Retrospective review of cerebral mycotic aneurysms in 26 patients: focus on treatment in strongly immunocompromised patients with a brief literature review. AJNR Am J Neuroradiol. 2013. 34: 823-7
3. Chapot R, Houdart E, Saint-Maurice JP, Aymard A, Mounayer C, Lot G. Endovascular treatment of cerebral mycotic aneurysms. Radiology. 2002. 222: 389-96
4. Cho SM, Rice C, Marquardt RJ, Zhang LQ, Khoury J, Thatikunta P. Magnetic Resonance Imaging Susceptibility-Weighted Imaging Lesion and Contrast Enhancement May Represent Infectious Intracranial Aneurysm in Infective Endocarditis. Cerebrovasc Dis. 2017. 44: 210-216
5. Ducruet AF, Hickman ZL, Zacharia BE, Narula R, Grobelny BT, Gorski J. Intracranial infectious aneurysms: A comprehensive review. Neurosurg Rev. 2010. 33: 37-46
6. Esenkaya A, Duzgun F, Cinar C, Bozkaya H, Eraslan C, Ozgiray E. Endovascular treatment of intracranial infectious aneurysms. Neuroradiology. 2016. 58: 277-84
7. Fukuda W, Daitoku K, Minakawa M, Fukui K, Suzuki Y, Fukuda I. Infective endocarditis with cerebrovascular complications: timing of surgical intervention. Interact Cardiovasc Thorac Surg. 2012. 14: 26-30
8. Grandhi R, Zwagerman NT, Linares G, Monaco EA, Jovin T, Horowitz M. Onyx embolization of infectious intracranial aneurysms. J Neurointerv Surg. 2014. 6: 353-6
9. Hamisch CA, Mpotsaris A, Timmer M, Reiner M, Stavrinou P, Brinker G. Interdisciplinary treatment of intracranial infectious aneurysms. Cerebrovasc Dis. 2016. 42: 493-505
10. John S, Walsh KM, Hui FK, Sundararajan S, Silverman S, Bain M. Dynamic angiographic nature of cerebral mycotic aneurysms in patients with infective endocarditis. Stroke. 2016. 47: e8-10
11. Kume Y, Fujita T, Fukushima S, Shimahara Y, Matsumoto Y, Yamashita K. Intracranial mycotic aneurysm is associated with cerebral bleeding post-valve surgery for infective endocarditis. Interact Cardiovasc Thorac Surg. 2018. 27: 635-41
12. Matsubara N, Miyachi S, Izumi T, Yamanouchi T, Asai T, Ota K. Results and current trends of multimodality treatment for infectious intracranial aneurysms. Neurol Med Chir (Tokyo). 2015. 55: 155-62
13. Molinari GF, Smith L, Goldstein MN, Satran R. Pathogenesis of cerebral mycotic aneurysms. Neurology. 1973. 23: 325-32
14. Nonaka S, Oishi H, Tsutsumi S, Teranishi K, Tanoue S, Yasumoto Y. Endovascular therapy for infectious intracranial aneurysm: A report of four cases. J Stroke Cerebrovasc Dis. 2016. 25: e33-7
15. Park W, Ahn JS, Park JC, Kwun BD, Lee DH. Treatment strategy based on experience of treating intracranial infectious aneurysms. World Neurosurg. 2017. 97: 351-9
16. Peters PJ, Harrison T, Lennox JL. A dangerous dilemma: Management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis. 2006. 6: 74-8
17. Petr O, Brinjikji W, Burrows AM, Cloft H, Kallmes DF, Lanzino G. Safety and efficacy of endovascular treatment for intracranial infectious aneurysms: A systematic review and meta-analysis. J Neuroradiol. 2016. 43: 309-16
18. Singla A, Fargen K, Blackburn S, Neal D, Martin TD, Hess PJ. National treatment practices in the management of infectious intracranial aneurysms and infective endocarditis. J Neurointerv Surg. 2016. 8: 741-6
19. Zanaty M, Chalouhi N, Starke RM, Tjoumakaris S, Gonzalez LF, Hasan D. Endovascular treatment of cerebral mycotic aneurysm: A review of the literature and single center experience. Biomed Res Int. 2013. 2013: 151643