- Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States.
- Department of Neurology Baylor College of Medicine, Houston, Texas, United States.
- Department of Pathology, Baylor College of Medicine, Houston, Texas, United States.
DOI:10.25259/SNI_416_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alex Flores1, Ron Gadot1, Ibrahim Noorbhai2, Hayden Hall2, Kent Alan Heck3, Daniel Matthew Sholto Raper1, David Xu1, Patrick Karas1, Jacob J. Mandel2, Alexander Eli Ropper1. S-100-negative, GNA11 mutation-positive intramedullary meningeal melanocytoma of the thoracic spine: A radiographic challenge and histologic anomaly. 28-Jun-2021;12:315
How to cite this URL: Alex Flores1, Ron Gadot1, Ibrahim Noorbhai2, Hayden Hall2, Kent Alan Heck3, Daniel Matthew Sholto Raper1, David Xu1, Patrick Karas1, Jacob J. Mandel2, Alexander Eli Ropper1. S-100-negative, GNA11 mutation-positive intramedullary meningeal melanocytoma of the thoracic spine: A radiographic challenge and histologic anomaly. 28-Jun-2021;12:315. Available from: https://surgicalneurologyint.com/surgicalint-articles/10921/
Abstract
Background: Intramedullary melanocytomas are exceedingly rare and their management is largely based on case reports and small clinical series. They have characteristic imaging and histologic findings that can aid in their diagnosis. Genetic testing may be required for definitive diagnosis and management guidance in ambiguous cases.
Case Description: We present the case of a thoracic intramedullary meningeal melanocytoma in a patient unable to undergo an MRI.
Conclusion: This is the first reported S-100-negative case with genetic testing to support the diagnosis of a rare intramedullary melanocytoma.
Keywords: GNA11 mutation, Intramedullary spinal tumor, Melanocytoma, S-100
INTRODUCTION
Meningeal melanocytomas are extremely uncommon tumors of the central nervous system (CNS) derived from leptomeningeal melanocytes, with only a few published case reports describing their incidence, presentation, and management. The first report in the English literature from 1972 along with a few following reports describes the tumors as occurring along the spine and posterior fossa with peak incidence around the 5th decade of life.[
CLINICAL PRESENTATION
A 69-year-old male with atrial fibrillation, history of cardiac arrest status with subsequent implantable cardioverter defibrillator (ICD) placement, and chronic kidney disease presented to the hospital with subacute progressive bilateral leg weakness, a vague lower thoracic sensory level, and both bowel and bladder dysfunction. Beginning 6 months before this, the patient reported loss of temperature sensation in his waist and legs and slowly developed bilateral leg weakness. Weeks before admission, he began experiencing progressive urinary and bowel retention and incontinence. He underwent an extensive outpatient workup including electromyography (EMG), lumbar puncture, and lumbar CT myelogram. EMG and CT myelogram were unremarkable. The only notable finding on initial evaluation was an elevated cerebrospinal fluid protein of 150 mg/dL (anti-NMO, anti-MOG, oligoclonal bands, and ACE all within normal limits). In an attempt to obtain an MRI, his ICD was removed, but unfortunately, the cardiac leads could not be completely recovered. Since an MRI could not be obtained, he was empirically started on dexamethasone, which did not improve his symptoms.
At time of admission, he had 4+/5 strength in all right lower extremity muscle groups, 0/5 L hip flexion, 2/5 L knee extension, decreased sensation below the T10 dermatome, bilateral patellar hyperreflexia, and extensor plantar reflexes bilaterally.
Hospital management
Given the subacute presentation of paraparesis with a sensory level, without obvious diagnosis from the spinal fluid, there was a clinical suspicion for a dural arteriovenous fistula. Spinal angiogram was performed, remarkable only for midline subtle vascular congestion at T9 within the cord parenchyma. Intravenous methylprednisolone was begun for concern for transverse myelitis with modest strength improvement and subsequently plasmapheresis with equivocal improvement.
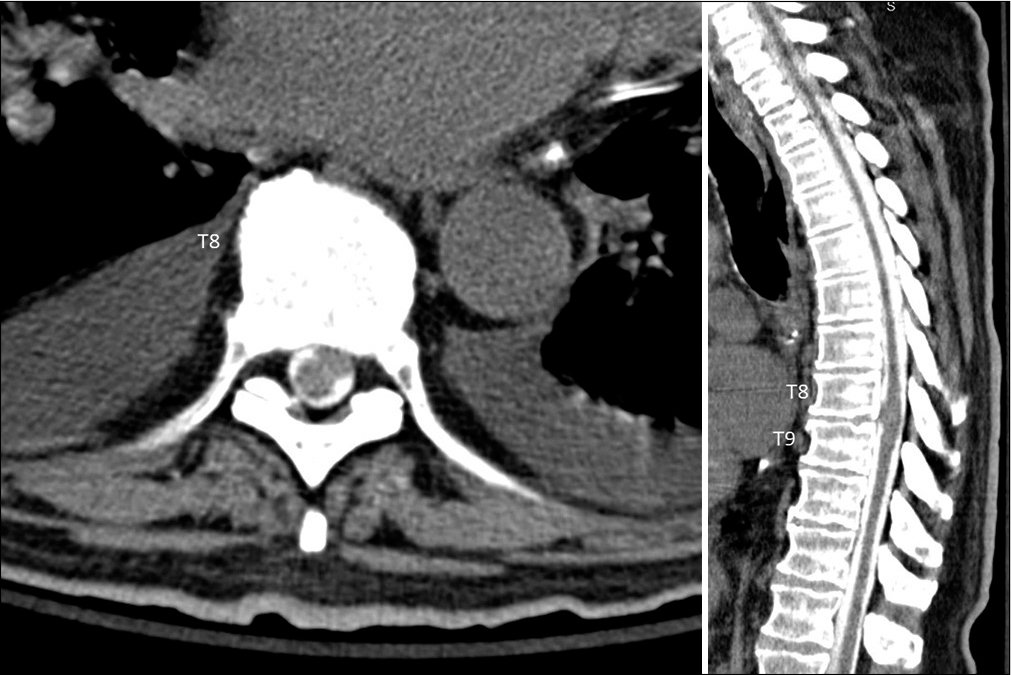
A thoracic CT myelogram was performed revealing what appeared to be an intradural, extramedullary lobulated lesion in the ventral left canal, presumed to be either a superiorly migrated disc extrusion, or an intradural extramedullary spinal tumor. The mass resulted in moderate to severe left-sided central canal stenosis contouring and displacing the cord to the right of midline [
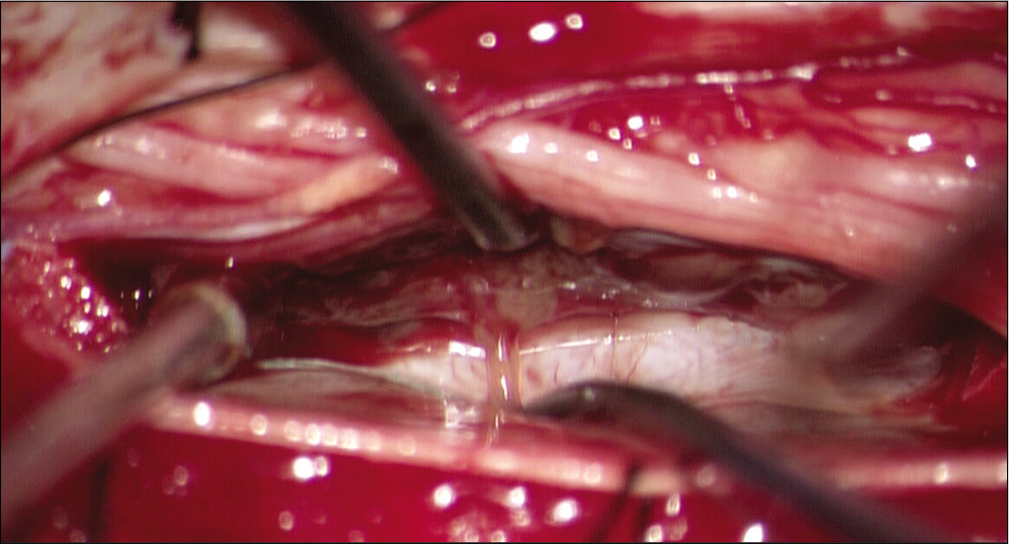
Given the patient’s lack of improvement to empiric treatment and the lack of a diagnosis, he ultimately underwent surgery for biopsy and resection of the mass. This was performed through a midline T8-9 laminectomy and intradural exploration. Intraoperatively, the tumor was located in the lateral subarachnoid space, but not emanated from or attached to the inside of the dura. The mass was originating within the cord parenchyma and had extruded out of the left lateral border of the spinal cord into the intradural, extramedullary space [
Pathology of the mass was consistent with a melanocytic tumor, possibly melanoma. Analysis of permanent sample revealed a proliferation of tumor cells found to be relatively bland with no necrosis identified. Melanin production was multifocal. Immunoperoxidase stains were diffusely positive for melanoma cocktail (HMB-45/Tyrosinase/MART-1) but negative for S-100. Immunoperoxidase stains for GFAP, AE1/3 were negative. The Ki-67 proliferation index was found to be no higher than 4.1%. Given the very rare mitoses, relatively low proliferation index, and cytologically bland histology, a primary meningeal melanocytoma was favored, but a metastatic melanoma to the spine or primary malignant melanoma could not be excluded.
An outside hospital dermatopathological conference reviewed permanent sections of the tumor and favored diagnosis of melanoma citing conspicuous nuclear and cellular morphologic features as well as areas of focal necrosis and increased proliferation index. Repeat analysis confirmed morphological and immunohistochemical features further supporting intermediate-to-high-grade primary meningeal melanocytoma versus melanoma [
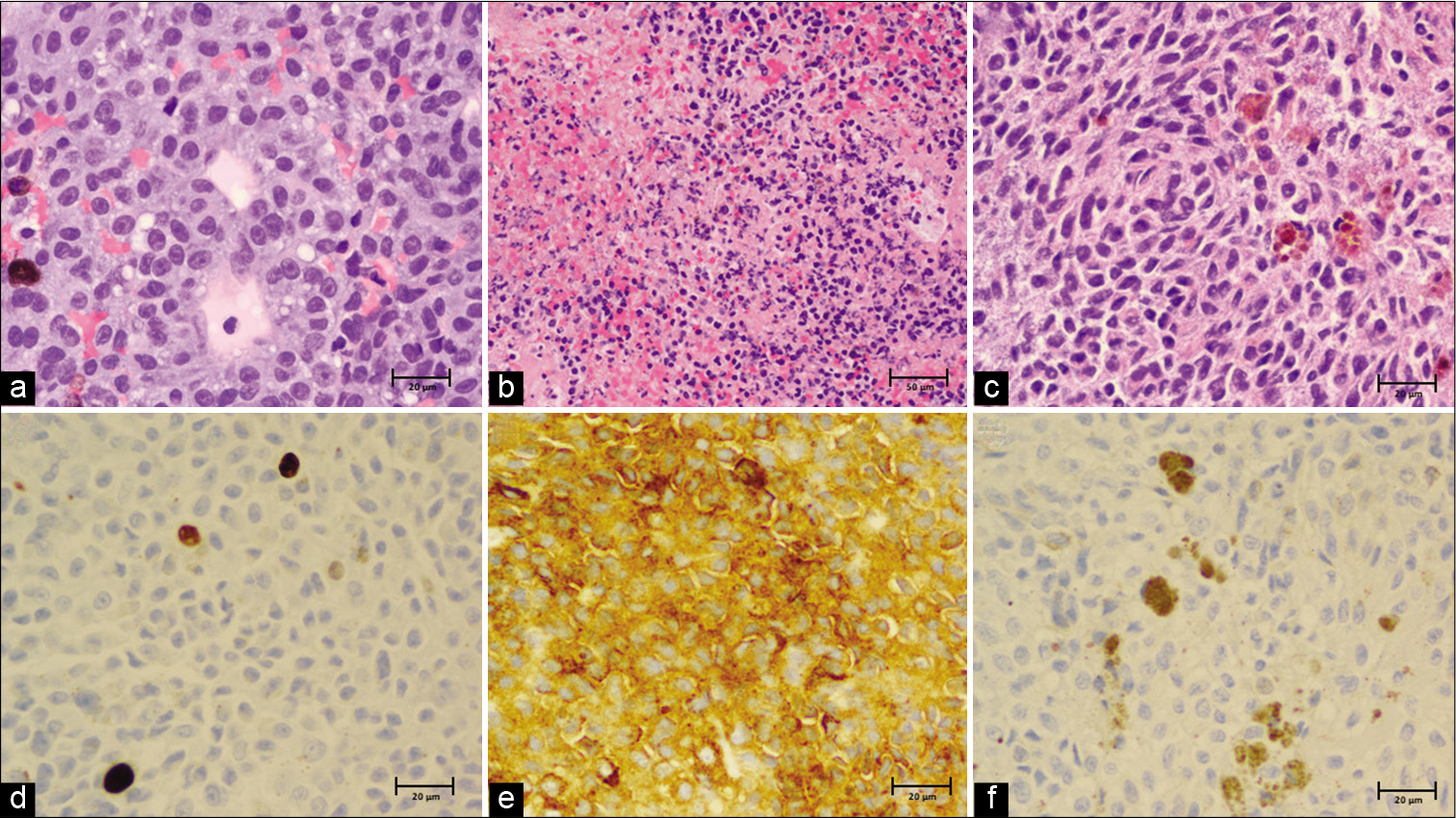
Figure 3:
Histopathology. (a) Hematoxylin and eosin (H&E) stain, ×40 and (b) ×20 showing a solid proliferation of atypical epithelioid cells with scattered pigmented melanophages amidst fibroconnective tissue. The samples are richly cellular with round to ovoid nuclei and prominent nucleoli. There are areas of necrosis with associated inflammation and hemorrhage. (c) Few spindle-shaped nests of cells admixed with melanophages were noted, ×40. (d) Ki-67 (MIB-1) index seen to be 1.5% with all other areas on ×40 found to be <4%. (e) HMB45/ Tyrosinase/MART-1 immunoperoxidase stain diffusely positive, ×40. (f) S-100 immunoperoxidase stain negative with interspersed dusty brown melanin pigment, ×40.
Postoperative workup for an extraspinal primary or metastatic melanoma including dermatological, otolaryngological, and ophthalmological examinations as well as a CT brain/spine/abdomen/pelvis without contrast and PET-CT scan was unremarkable. The patient was discharged to inpatient rehabilitation and discharged from rehabilitation after 3 weeks. Despite worsening strength in the left leg immediately postoperatively, he ultimately regained strength in the left leg to his preoperative baseline with improved right leg strength. No decision has been made regarding pursuing adjuvant radiotherapy, though it has been recommended. Genetic testing (Tempus, Chicago, IL) on permanent sample revealed a GNA11 p.Q209L missense gain-of-function (GOF) mutation and was without mutation in BRAF, NRAS, or KIT genes favoring the diagnosis of meningeal melanocytoma.
DISCUSSION
Melanocytoma is a rare primary spinal tumor with an annual incidence of 1/10 million, representing <0.1% of all CNS tumors.[
Although presentation depends on location of the tumor, workup invariably leads to neuroimaging. MRI represents the modality of choice, especially given the characteristic MRI signature of the melanin pigment.[
Between the WHO criteria and a proposition by Brat et al., melanocytomas are divided histologically into low-grade melanocytomas, intermediate-grade tumors, and malignant melanomas.[
Concerning the possibility that this tumor could have been a melanoma, much attention was paid to its histopathology. While the tumor stained positively for melanoma cocktail, it stained negatively for S-100 which has been found to be the most sensitive marker for melanomas.[
In histologically challenging cases of melanocytomas such as this, molecular profiling has been shown to help differentiate between malignant melanoma and lower-grade primary melanocytomas, with the presence of GNAQ mutation in the absence of typical mutations of cutaneous melanomas including BRAF and NRAS suggesting primary CNS tumor.[
Gross total resection is the initial treatment of choice for these tumors as survival is better and local control rates have been shown to be 4 times higher if complete resection is achieved.[
More cases are required to obtain accurate recurrence rates, but authors have noted recurrences to be common.[
CONCLUSION
We report on a radiologically and histologically challenging case of a thoracic intramedullary melanocytic neoplasm that was determined to be an intermediate-to-high-grade melanocytoma given immunohistochemical and molecular analysis. Although rarely used since the advent of MRI, CT myelogram should be considered as a diagnostic means for a differential that includes expansile intramedullary lesions in the setting of contraindications to MRI. In the absence of confirmed primary melanotic tumors, mutational analysis of suggestive genetic loci including GNA11, GNAQ, BRAF, NRAS, and cKIT can be useful in definitive diagnosis and management.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aisner DL, Maker A, Rosenberg SA, Berman DM. Loss of S100 antigenicity in metastatic melanoma. Hum Pathol. 2005. 36: 1016-9
2. Argenyi ZB, Cain C, Bromley C, Nguyen AV, Abraham AA, Kerschmann R. S-100 protein-negative malignant melanoma: Fact or fiction? A light-microscopic and immunohistochemical study. Am J Dermatopathol. 1994. 16: 233-40
3. Biernacka A, Linos KD, DeLong PA, Suriawinata AA, Padmanabhan V, Liu X. A case of S-100 negative melanoma: A diagnostic pitfall in the workup of a poorly differentiated metastatic tumor of unknown origin. Cytojournal. 2016. 13: 21
4. Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol. 1999. 23: 745-54
5. Brat DJ, Parisi JE, Kleinschmidt-DeMasters BK, Yachnis AT, Montine TJ, Boyer PJ.editors. Surgical neuropathology update: A review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition. Arch Pathol Lab Med. 2008. 132: 993-1007
6. de Potter P, Shields CL, Eagle RC, Shields JA, Lipkowitz JL. Malignant melanoma of the optic nerve. Arch Ophthalmol. 1996. 114: 608-12
7. Dorwal P, Mohapatra I, Gautam D, Gupta A. Intramedullary melanocytoma of thoracic spine: A rare case report. Asian J Neurosurg. 2014. 9: 36-9
8. Dubey A, Kataria R, Sardana VR. Intramedullary melanocytoma of the cervicothoracic cord: Case report and review of literature. Asian J Neurosurg. 2018. 13: 478-81
9. Eskandari R, Schmidt MH. Intramedullary spinal melanocytoma. Rare Tumors. 2010. 2: e24
10. Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, DeMarini DJ. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: A phase 1 dose-escalation trial. Lancet Oncol. 2012. 13: 782-9
11. Hoffmann M, Koelsche C, Seiz-Rosenhagen M, Mai S, Lohr F, Reuss D. The GNAQ in the haystack: Intramedullary meningeal melanocytoma of intermediate grade at T9-10 in a 58-year-old woman. J Neurosurg. 2016. 125: 53-6
12. Horn EM, Nakaji P, Coons SW, Dickman CA. Surgical treatment for intramedullary spinal cord melanocytomas. J Neurosurg Spine. 2008. 9: 48-54
13. Koelsche C, Hovestadt V, Jones DT, Capper D, Sturm D, Sahm F. Melanotic tumors of the nervous system are characterized by distinct mutational, chromosomal and epigenomic profiles. Brain Pathol. 2015. 25: 202-8
14. Küsters-Vandevelde HV, Klaasen A, Küsters B, Groenen PJ, van Engen-van Grunsven IA, van Dijk MR. Activating mutations of the GNAQ gene: A frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol. 2010. 119: 317-23
15. Limas C, Tio FO. Meningeal melanocytoma (“melanotic meningioma”). Its melanocytic origin as revealed by electron microscopy. Cancer. 1972. 30: 1286-94
16. Litofsky NS, Zee CS, Breeze RE, Chandrasoma PT. Meningeal melanocytoma: Diagnostic criteria for a rare lesion. Neurosurgery. 1992. 31: 945-8
17. Merhemic Z, Stosic-Opincal T, Thurnher MM. Neuroimaging of spinal tumors. Magn Reson Imaging Clin N Am. 2016. 24: 563-79
18. O’Brien TF, Moran M, Miller JH, Hensley SD. Meningeal melanocytoma. An uncommon diagnostic pitfall in surgical neuropathology. Arch Pathol Lab Med. 1995. 119: 542-6
19. Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008. 35: 433-44
20. Parish AJ, Nguyen V, Goodman AM, Murugesan K, Frampton GM, Kurzrock R. GNAS, GNAQ, and GNA11 alterations in patients with diverse cancers. Cancer. 2018. 124: 4080-9
21. Rades D, Heidenreich F, Tatagiba M, Brandis A, Karstens JH. Therapeutic options for meningeal melanocytoma. Case report. J Neurosurg. 2001. 95: 225-31
22. Rades D, Schild SE. Dose-response relationship for fractionated irradiation in the treatment of spinal meningeal melanocytomas: A review of the literature. J Neurooncol. 2006. 77: 311-4
23. Rades D, Schild SE, Tatagiba M, Molina HA, Alberti W. Therapy of meningeal melanocytomas. Cancer. 2004. 100: 2442-7
24. Rades D, Tatagiba M, Brandis A, Dubben HH, Karstens JH. The value of radiotherapy in treatment of meningeal melanocytoma. Strahlenther Onkol. 2002. 178: 336-42
25. Shinohara MM, Deubner H, Argenyi ZB. S100, HMB-45, and melan-a negative primary melanoma. Dermatol Online J. 2009. 15: 7
26. Wagner F, Berezowska S, Wiest R, Gralla J, Beck J, Verma RK. Primary intramedullary melanocytoma in the cervical spinal cord: Case report and literature review. Radiol Case Rep. 2015. 10: 1010
27. Zubovits J, Buzney E, Yu L, Duncan LM. HMB-45, S-100, NK1/C3, and MART-1 in metastatic melanoma. Hum Pathol. 2004. 35: 217-23