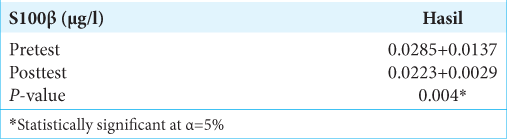- Department of Neurosurgery, Dr. Moewardi General Hospital, Faculty of Medicine University of Sebelas Maret, Surakarta, Indonesia,
- Faculty of Medicine University of Sebelas Maret, Department of Neurosurgery, Dr. Moewardi General Hospital, University of Sebelas Maret Hospital, Surakarta, Indonesia,
- Department of Surgery, Dr. Moewardi General Hospital, Faculty of Medicine University of Sebelas Maret, Surakarta, Indonesia.
Correspondence Address:
Ferry Wijanarko, Department of Neurosurgery, Dr. Moewardi General Hospital, Faculty of Medicine University of Sebelas Maret, Surakarta, Central Java, Indonesia.
DOI:10.25259/SNI_294_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ferry Wijanarko1, Untung Alifianto1, Hanis Setyono1, Geizar Arsika Ramadhana1, Affan Adib Sungkar2, Ikhdin Saadhi3, Dea Alberta Setiawati3. S100β protein levels as a parameter to assess the clinical development of adult patients with mild traumatic brain injury in Dr. Moewardi Public Hospital, Surakarta. 12-Jul-2021;12:342
How to cite this URL: Ferry Wijanarko1, Untung Alifianto1, Hanis Setyono1, Geizar Arsika Ramadhana1, Affan Adib Sungkar2, Ikhdin Saadhi3, Dea Alberta Setiawati3. S100β protein levels as a parameter to assess the clinical development of adult patients with mild traumatic brain injury in Dr. Moewardi Public Hospital, Surakarta. 12-Jul-2021;12:342. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10966
Abstract
Background: Mild traumatic brain injury (mTBI) is a health problem with an increasing incidence in many developed countries. The standard for examining mTBI is a CT scan, but it is costly, is not always available in all hospitals, and carries a risk of radiation. Meanwhile, S100β is a protein component produced by central nervous system cells. This study aims to determine the presence of changes in S100β protein in adult patients with mTBI during treatment as an alternative to examination.
Methods: This research is an analytic observational quantitative study with a cross-sectional study approach to investigate changes in S100β protein levels in blood serum using the ELISA method of mTBI patients in the first 3 h posttrauma (pretest) and treatment on day 1 (27 h posttrauma/posttest). The research sample consisted of 22 people. This research was conducted in the Surgery Section, Sub-Division of Neurosurgery, Dr. Moewardi Public Hospital, during September–December 2019. The data were then analyzed using a discrimination test (comparing t-test means) and a nonparametric test (Wilcoxon).
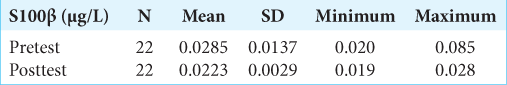
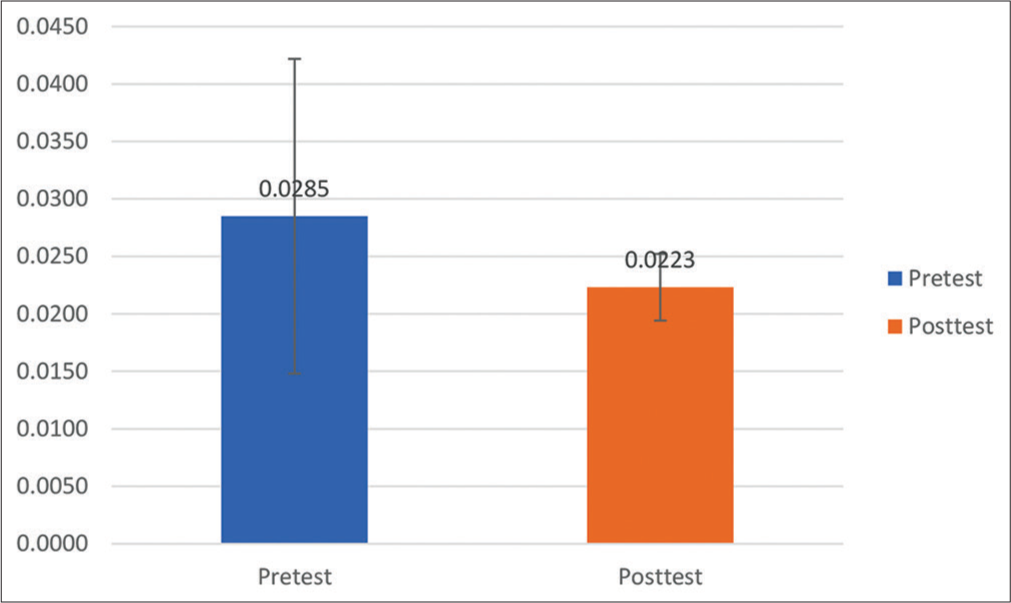
Results: There was a significant difference in mean S100β change between the pretest and posttest treatments. The S100β examination results at posttest decreased to 0.0223 + 0.0029 μg/l or decreased S100β by 21.7% after treatment. Previously, it was known that the mean of S100β at pretest was 0.0285 + 0.0137 μg/l.
Conclusion: There was a significant change in S100β protein levels at each examination time. Changes in S100β levels that occurred were in the form of decreased levels from 3 h to 27 h posttrauma. Thus, S100β protein can be used as a parameter to assess the clinical development of adult patients with mTBI. Moreover, none of the patients with an S100β value >0.1 μg/l was found to be the cutoff value set by SNC in adult patients with mTBI for head CT scan.
Keywords: Head CT scan, Mild traumatic brain injury, S100β
INTRODUCTION
Brain injury is serious problem in developing countries. Most (up to 95%) of head injuries are classified as mild traumatic brain injury (mTBI), commonly defined as Glasgow Coma Scale (GCS) 13–15 with the presence of certain risk factors such as loss of consciousness and/or amnesia.[
Research data related to brain injury epidemiology to date are still mainly obtained from various developed countries, such as the United States and the European Union. Research data from the European Union found that brain injury incidence reaches 2.5 million cases each year, 90% of which are cases of mTBI.[
S100β is a dimeric astroglial of approximately 21kD, consisting of ββ-chains and belonging to a multigenic family of calcium-binding protein expressing a great variety of homodimeric and heterodimeric protein.[
MATERIALS AND METHODS
This study was an analytic observational quantitative study with a cross-sectional study approach to study changes in S100β protein levels in the blood serum of mTBI patients during the first 3 h of posttrauma and day 1 treatment (27 h posttrauma) without any further follow-up. The patient treated in neurosurgical ward for a day. The research was conducted at the Surgery Section, Sub-Division of Neurosurgery, Dr. Moewardi Public Hospital, during September–December 2019.
The study population included all patients with an mTBI without surgery who came to the surgical emergency room and were treated in the ward by the Sub-Division of Neurosurgery, Dr. Moewardi Public Hospital, Surakarta. The sampling technique employed purposive sampling, then adjusted to the inclusion and exclusion criteria to obtain a total sample size of 22. The inclusions criteria were isolated mild brain injury, mongoloid race, age more than 18 years old, approval by the patients, and arrival in emergency department <3 h after accident. The exclusion criteria were posttraumatic seizures, focal neurological deficit, clinical sign of depressed or basal skull fracture, shunt-treated hydrocephalus, anticoagulation therapy, coagulation disorders, and anti-platelet medication.
The samples were examined for the S100β protein level in the blood serum 3 h from the event’s onset. Then, in treatment 27 h after the onset of the event, they were rechecked. The blood samples were taken from the patient’s peripheral blood, which was then processed in the clinical laboratory. S100β levels were measured by the ELISA method.
The patients were obtained head CT scan 6 h after accident, but if the patient experienced a decrease in consciousness during the observation (before 6 h), a head CT scan performing immediately. If the bleeding was found in the head CT scan, a craniotomy surgery was performed; then, the patient was dropped out as the study subject.
All data collected were analyzed by statistical analysis with a discrimination test comparing between t-test means if they were normally distributed. However, if the distribution was not normal, then a nonparametric test (Wilcoxon) would be utilized.
RESULTS
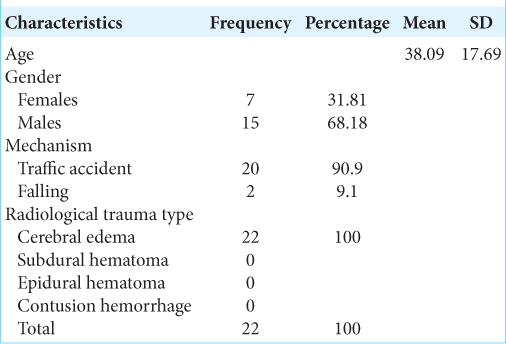
In this study, the research subject characteristics based on age obtained a mean age of 38.09 years, with a standard deviation of 17.69 years, a minimum age of 18 years, and a maximum age of 68 years. There were seven female subjects (31.81%) and 15 male subjects (68.18%). Furthermore, the mechanism of trauma in 20 (90.9%) research subjects was traffic accident victims, while 2 (9.1%) subjects were victims of the fall in the household environment. The head CT scan in all study subjects indicated cerebral edema with description such as cisterna closing, gyrus and sulcus disappearing, no mid line shift, and absence of intracranial bleeding lesions. The data are listed in [
The examination of S100β protein levels in blood serum was in the first 3 h posttrauma (pretest), then was rechecked in treatment day 1 (27 h postevent/posttest). The following results were obtained:
Based on [
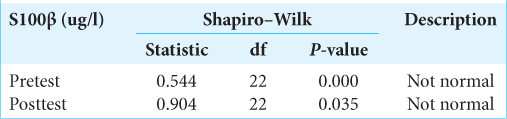
The data were then tested for normality using the Shapiro– Wilk test. The research data are said to be normally distributed if P > 0.05 was considered. The Shapiro–Wilk test on the S100β data obtained the following results:
[
Based on the Wilcoxon test results in [
DISCUSSION
Serum biomarkers can be used as an additional mean to identify patients at risk for intracerebral lesion, marking them for further analysis. After the onset of intracranial lesions, S100β is immediately released from the damaged glial cells into the circulatory system. The result from multicenter study undoubtedly shows at a high-level statistical reliability that measurement of S100β serum concentrations provides substantial information for the management of mTBI patients and, in addition to conventional clinical decision rules, might allow the reduction of head CT scans by approximately 30% of cases.[
The general characteristics of this study’s subjects were that the majority were male in their productive age, and the majority were victims of traffic accidents. It is in accordance with a study’s results, stating that the most common cause of brain injury is traffic accidents and often occurs in men, especially at a young age who have high mobility.[
S100β has a limit of 0.10 μg/l, with a sensitivity value of 95% and a specificity of 31%. Particularly, it means that among patients with a serum level of ≥0.1 μg/l, 12% exhibited intracranial pathological findings. Among those with serum levels <0.1 μg/l, only 1.5% had such intracranial lesions, which was not clinically significant in this study. If this risk is accepted, a head CT scan can be eliminated in patients with S100β levels <0.1 μg/l. Hence, the question arises for the doctor to decide whether this risk is acceptable; the small probability of loss of intracranial lesions must be weighed against the cost and patient radiation’s safety.[
Based on the research results of the S100β examination before treatment (pretest), the mean was 0.0285 + 0.0137 μg/l. At 27 h after treatment, the patient received the S100β test results that decreased to 0.0223 + 0.0029 μg/l or experienced a 21.7% decrease in S100β after treatment. The statistical test obtained P = 0.004 (P < 0.05), signifying that there was a significant difference in mean S100β change between pretest and posttest treatments (27 h postevent). However, in the S100β examination results, none of the patients with an S100β value >0.1 μg/l was found to be the cutoff value for head CT scan in adult patients with mTBI by Scandinavian Neurotrauma Committee (SNC).
The cutoff values set by the SNC come from studies involving a large proportion of the Caucasian population. This value may not account for the same in a race of color, which affects the specificity of S100β more (e.g. more false positives) and, therefore, theoretically, reduces the need for a head CT scan of S100β in the non-Caucasian population.[
The current analysis showed that measuring S100β improved the prediction of scanning abnormalities in the GCS 14– 15 groups so that the S100β measurement application in guidelines for mTBI management could avoid the use of a head CT scan. It will reduce management costs and radiation effects significantly.[
CONCLUSION
Based on the research results conducted on 22 patients with mTBI, it could be concluded that there was a significant change in S100β protein levels at each examination time. Changes in S100β levels that occurred were in the form of decreased levels from 3 h to 27 h posttrauma. Thus, S100β protein can be used as a parameter to assess the clinical development of adult patients with mTBI. Moreover, none of the patients with an S100β value >0.1 μg/l was found to be the cutoff value set by SNC in adult patients with mTBI for head CT scan. The cost is much lower price.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Biberthaler P, Linsenmeier U, Pfeifer KJ, Kroetz M, Mussack T, Kanz KG. Serum S-100B concentration provides additional information fot the indication of computed tomography in patients after minor head injury: A prospective multicenter study. Shock. 2006. 25: 446-53
2. Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM. Traumatic brain injuries. Nat Rev Dis Primers. 2016. 2: 16084
3. Calcagnile O, Anell A, Unden J. The addition of S100B to guidelines for management of mild head injury is potentially cost saving. BMC Neurol. 2016. 16: 200
4. Feigin VL, Theadom A, Barker-Collo S, Starkey NJ, McPherson K, Kahan M. Incidence of traumatic brain injury in New Zealand: A population-based study. Lancet Neurol. 2013. 12: 53-64
5. Feinstein J, Stahl KD.editors. Acute Care Surgery and Trauma: Traumatic Brain Injury. Texas, USA: Informa; 2009. p. 72
6. Keisuke K, Liu CY, Merkel SF, Ramirez SH, Tiarney RT, Langford D. Blood biomarkers for brain injury: What are we measuring?. Neurosci Biobehav Rev. 2016. 68: 460-73
7. Muller K, Townend W, Biasca N. S100B serum levels predict computed tomography finding after minor head injury. J Trauma. 2007. 62: 1452-6
8. Oris C, Bruno P, Durif J, Simon-Pimmel J, Castellani C, Manzano S. The biomarker S100B and mild traumatic brain injury: A meta-analysis. Pediatrics. 2018. 141: 209-25
9. Quentin D, François L, Benjamin K, Raphaël L, Jean-François A, Thierry S. evaluation of the Roche® elecsys and the Diasorin® liaison s 100 kits in the management of mild head injury in the emergency room. Clin Biochem. 2017. 13: 11-23
10. Skandsen T, Einersen CE, Normann I. The epidemiology of mild traumatic brain injury: The Trondheim MTBI follow-up study. Scand J Trauma Resusc Emerg Med. 2018. 26: 34
11. Sorci G, Riuzzi F, Arcuri C, Tubaro C, Bianchi R, Giambanco I. S100β protein in tissue development, repair and regeneration. World J Biol Chem. 2013. 4: 1-12
12. Thelin EP, Johannesson L, Nelson DW, Bellander BM. S100B is an important outcome predictor in traumatic brain injury. J Neurotrauma. 2013. 30: 519-28
13. Thelin EP, Nelson DW, Bellander BM. A review of the clinical utility of serum S100β protein levels in the assessment of traumatic brain injury. Acta Neurochir (Wien). 2017. 159: 209-25
14. Unden L, Calcagnile O, Unden J, Reinstrup P, Bazarian J. Validation of Scandinavian guidelines for initial management of minimal, mild, and moderate traumatic brain injury in adults. BMC Med. 2015. 13: 292
15. Kelmendi FM, Morina AA, Mekaj AY, Blyta A, Alimehmeti R, Dragusha S. Serum S100B levels can predict computed tomography findings in paediatric patients with mild head injury. Biomed Res Int. 2018. 7: 1