- Department of Neurosurgery, Riverside University Health System, Riverside, California, United States.
DOI:10.25259/SNI_244_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kevin Ray, Mark Krel, Jacob Bernstein, Samir Kashyap, Ajay Ananda. Safety of the transventricular approach to deep brain stimulation: A retrospective review. 04-Oct-2019;10:192
How to cite this URL: Kevin Ray, Mark Krel, Jacob Bernstein, Samir Kashyap, Ajay Ananda. Safety of the transventricular approach to deep brain stimulation: A retrospective review. 04-Oct-2019;10:192. Available from: http://surgicalneurologyint.com/surgicalint-articles/9685/
Abstract
Background: Anatomically, deep brain stimulation (DBS) targets such as the ventral intermediate and subthalamic nucleus are positioned such that the long axis of the nucleus is often most accessible through a transventricular trajectory. We hypothesize that using this trajectory does not place patients at increased risk of neurologic complications.
Methods: A series of 206 patients at a single institution between 2000 and 2017 were reviewed. All patients had a confirmed transventricular trajectory and their clinical course was reviewed to assess neurologic complication rates in the postoperative period.
Results: The average length of hospital stay was 2.4 days. The most common neurologic complication was altered mental status in 1.2% of cases (four patients). This was followed by seizure in 0.6% of cases (two patients). No patients had ischemic stroke or postoperative hemiparesis. There were two mortalities in this series, one with lobar hemorrhage contralateral from the surgical site and one with a thalamic hemorrhage. There was only one confirmed intraventricular hemorrhage postoperatively; however, this was clinically asymptomatic.
Conclusion: Although the total incidence of intraventricular or intracerebral hemorrhage cannot be reliably assessed from this data set, the low incidence of neurologic complications challenges the notion that DBS electrode trajectories that transgress the ventricle significantly increase the risk of complications.
Keywords: Deep brain stimulation, Functional, Movement disorders, Parkinson’s disease, Transventricular, Tremor
INTRODUCTION
Deep brain stimulation (DBS) for the treatment of diseases such as Parkinson’s, dystonia, and essential tremor has increased in popularity since its introduction in the early nineties. Conventionally, care has been taken to avoid electrode trajectories that traverse the lateral ventricle due to the risk of neurologic complications such as intraventricular hemorrhage, seizure, postoperative confusion, and reduced accuracy of electrode placement.[
METHODS
Patient selection and variables assessed
This was a retrospective analysis of 206 patients who underwent DBS for movement disorders between 2000 and 2017 at a single institution. A total of 206 patients underwent 326 DBS procedures [
Operative technique
All patients were placed in a Leksell frame on the day of surgery with a local anesthetic (1% lidocaine with epinephrine + 0.25%–0.5% bupivacaine) and CT stereotactic images were obtained. The intended target and trajectory were identified and planned using StealthStation Navigation™ (Medtronic) with an emphasis on avoiding sulci and any obvious vasculature. A medial approach with an angle of 2–10° in the coronal plane was favored in most cases, regardless of whether or not the trajectory would pass through the lateral ventricles. Transventricular trajectories for VIM and STN placement are shown in
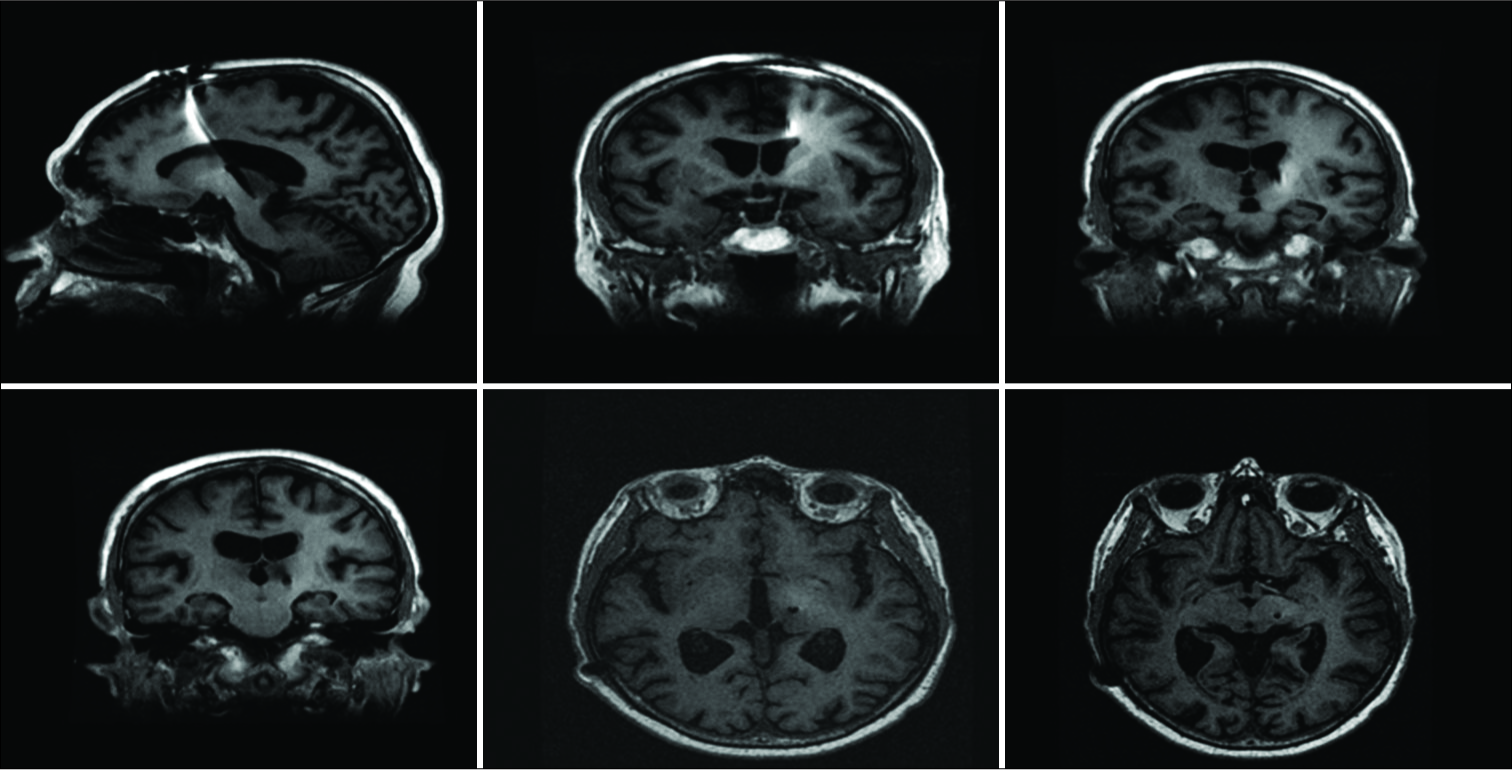
Figure 1:
Ventral intermediate (VIM) trajectory. T1 magnetic resonance imaging showing trajectory of the left VIM electrode. Top left – sagittal view of electrode at target, top middle – coronal view of electrode entering left lateral ventricle, top right – coronal view of electrode exiting left lateral ventricle, bottom right – axial view of electrode at target.
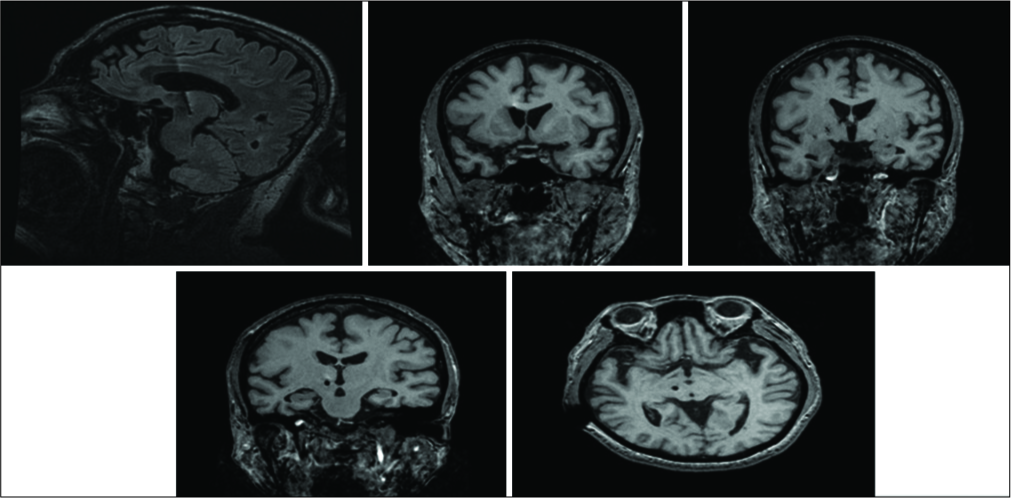
Figure 2:
Subthalamic nucleus (STN) trajectory. T1 magnetic resonance imaging showing trajectory of the right STN electrode. Top left – sagittal view of electrode at target, top middle – coronal view with electrode entering right lateral ventricle, top right – electrode exiting ventricle, bottom middle – axial view of electrode at target.
The vast majority of procedures were performed without sedation to allow for intraoperative stimulation testing. Patients were placed in a chaise lounge position and 14 mm precoronal burr holes were drilled based on the planned trajectory. The dura was coagulated, incised, and a microstimulator was introduced. The burr holes were packed with GelFoam® (Pfizer) to minimize loss of CSF. Microelectrode recording was performed in STN or GPi lead placements. Once adequate electrodeposition was obtained, permanent electrodes were inserted, and patients were retested to ensure favorable positioning before closing. Intraoperative testing was assisted by neurologists and neurology physician assistants present in the operating room. Following implantation of the DBS electrodes, the stereotactic frame was removed, and the patients were immediately anesthetized and prepped for implantation of the impulse generator or connection to an existing generator. In rare cases, some patients were deemed too unstable to continue.
RESULTS
Patient population
Over a 17-year period (2000–2017), 206 patients (137 males and 69 females) were included with 326 confirmed transventricular electrodes. The average age was 64 years old. The indications for implantation were Parkinson’s disease (156 patients, 246 electrodes), essential tremor (47 patients, 77 electrodes), dystonia (2 patients, patients), and Tourette syndrome (1 patient, 1 electrode) [
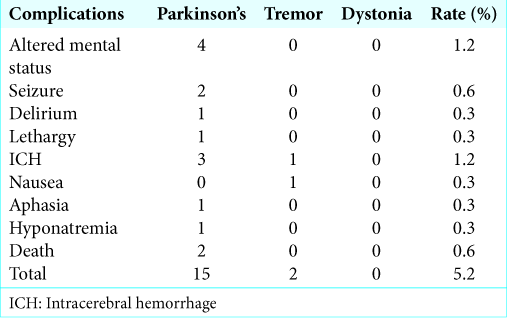
Complications
The total complication rate in our study was 5.6%, with higher complication rates for patients with Parkinson’s (6.1%) versus essential tremor (2.6%), dystonia (0%), or Tourette’s (0%) [
DISCUSSION
In our series, we report comparable or lower complication rates utilizing a transventricular trajectory for DBS. Our frequency of intracerebral hemorrhage (ICH) (1.2%) is lower than the average rate of 3% reported in literature; however, ranges of 1.2–3.6% have been reported.[
Headache was commonly reported though this is an expected finding and no patients in our study had severe headache documented beyond postoperative day 2. Seizures are an uncommon finding after DBS with a reported rate of 1.2% by Kenney et al. and 4.3% reported by Pouratian et al., in 2011.[
Risks of transventricular trajectory
The previous authors report an increased risk of complications such as IVH with transgressing the ventricle such as confusion and increased length of stay.[
Elias et al. identified 15 intracerebral adverse events of 113 transventricular lead placements though none of these complications were directly associated to ventricular punctures. They also found 5% rate of IVH, but this was clinically asymptomatic.[
Limitations
A major limitation of our study is the fact that not all patients receive immediate postoperative imaging to detect any potential intracranial hemorrhage. This limits the uniformity of our data analysis; however, we do not believe that this had any bearing on patient outcome or management as those patients who exhibited signs of neurologic dysfunction received appropriate imaging. Another limitation of our study exists with respect to accuracy of actual electrode placement compared to the intended target. Preoperative target plans were not routinely saved on the navigation software nor were immediate postoperative scans obtained for comparison in most cases. The previous authors have reported drift from the intended target due to loss of CSF or deflection of electrodes off ependymal lining of the ventricle although this was not found to be clinically significant.[
CONCLUSION
Our results challenge the notion that transventricular lead placement puts patients at a significantly higher risk for neurologic complications, particularly with respect to clinically significant intraventricular hemorrhage, postoperative confusion, or seizures. We found no clinically significant neurologic complications associated with a transventricular approach. Although the incidence of intraventricular hemorrhage is underreported in this study due to lack of routine postoperative imaging, it can be inferred that if intraventricular hemorrhage was present, it was not clinically relevant. Therefore, we believe that using a transventricular trajectory is safe and offers an optimal trajectory to maximize electrode placement in medial structures such as the STN or VIM. Prospective studies are recommended to help confirm these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Kaiser Permanente Sunset, Los Angeles, CA; Riverside University Health System, Moreno Valley, CA. No funding was required for this retrospective review.
References
1. Andrade-Souza YM, Schwalb JM, Hamani C, Eltahawy H, Hoque T, Saint-Cyr J. Comparison of three methods of targeting the subthalamic nucleus for chronic stimulation in parkinson’s disease. Oper Neurosurg. 2005. 56: 360-8
2. Ben-Haim S, Asaad WF, Gale JT, Eskandar EN. Risk factors for hemorrhage during microelectrode-guided deep brain stimulation and the introduction of an improved microelectrode design. Neurosurgery. 2009. 64: 754-62
3. Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of parkinson’s disease. Lancet Neurol. 2009. 8: 67-81
4. Bourne SK, Conrad A, Konrad PE, Neimat JS, Davis TL. Ventricular width and complicated recovery following deep brain stimulation surgery. Stereotact Funct Neurosurg. 2012. 90: 167-72
5. Elias WJ, Sansur CA, Frysinger RC. Sulcal and ventricular trajectories in stereotactic surgery. J Neurosurg. 2009. 110: 201-7
6. Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: Management and avoidance. J Neurosurg. 2014. 120: 132-9
7. Gologorsky Y, Ben-Haim S, Moshier EL, Godbold J, Tagliati M, Weisz D. Transgressing the ventricular wall during subthalamic deep brain stimulation surgery for parkinson disease increases the risk of adverse neurological sequelae. Neurosurgery. 2011. 69: 294-9
8. Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg. 2007. 106: 621-5
9. Khan S, Javed S, Park N, Gill SS, Patel NK. A magnetic resonance imaging-directed method for transventricular targeting of midline structures for deep brain stimulation using implantable guide tubes. Neurosurgery. 2010. 66: 234-7
10. Pouratian N, Reames DL, Frysinger R, Elias WJ. Comprehensive analysis of risk factors for seizures after deep brain stimulation surgery. Clinical article. J Neurosurg. 2011. 115: 310-5
11. Tabbal SD, Revilla FJ, Mink JW, Schneider-Gibson P, Wernle AR, de Erausquin GA. Safety and efficacy of subthalamic nucleus deep brain stimulation performed with limited intraoperative mapping for treatment of parkinson’s disease. Neurosurgery. 2007. 61: 119-27
12. Terao T, Takahashi H, Yokochi F, Taniguchi M, Okiyama R, Hamada I. Hemorrhagic complication of stereotactic surgery in patients with movement disorders. J Neurosurg. 2003. 98: 1241-6
13. Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB. Deep brain stimulation for movement disorders: Morbidity and mortality in 109 patients. J Neurosurg. 2003. 98: 779-84
14. Voges J, Waerzeggers Y, Maarouf M, Lehrke R, Koulousakis A, Lenartz D. Deep-brain stimulation: Long-term analysis of complications caused by hardware and surgery experiences from a single centre. J Neurol Neurosurg Psychiatry. 2006. 77: 868-72
15. Xiaowu H, Xiufeng J, Xiaoping Z, Bin H, Laixing W, Yiqun C. Risks of intracranial hemorrhage in patients with parkinson’s disease receiving deep brain stimulation and ablation. Parkinsonism Relat Disord. 2010. 16: 96-100
16. Zrinzo L, Foltynie T, Limousin P, Hariz MI. Reducing hemorrhagic complications in functional neurosurgery: A large case series and systematic literature review. J Neurosurg. 2012. 116: 84-94
17. Zrinzo L, van Hulzen AL, Gorgulho AA, Limousin P, Staal MJ, De Salles AA. Avoiding the ventricle: A simple step to improve accuracy of anatomical targeting during deep brain stimulation. J Neurosurg. 2009. 110: 1283-90