- Departments of Neurosurgery, Kurume University School of Medicine, Kurume, Fukuoka, Japan.
- Departments of Radiology, Kurume University School of Medicine, Kurume, Fukuoka, Japan.
Correspondence Address:
Nobuyuki Takeshige
Departments of Neurosurgery, Kurume University School of Medicine, Kurume, Fukuoka, Japan.
DOI:10.25259/SNI_182_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nobuyuki Takeshige, Takachika Aoki, Kiyohiko Sakata, Soushou Kajiwara, Tetsuya Negoto, Satoshi Nagase, Syuichi Tanoue, Yusuke Uchiyama, Masaru Hirohata, Toshi Abe, Motohiro Morioka. Sagittal diffusion-weighted imaging in preventing the false-negative diagnosis of acute brainstem infarction: Confirmation of the benefit by anatomical characterization of false-negative lesions. 20-Sep-2019;10:180
How to cite this URL: Nobuyuki Takeshige, Takachika Aoki, Kiyohiko Sakata, Soushou Kajiwara, Tetsuya Negoto, Satoshi Nagase, Syuichi Tanoue, Yusuke Uchiyama, Masaru Hirohata, Toshi Abe, Motohiro Morioka. Sagittal diffusion-weighted imaging in preventing the false-negative diagnosis of acute brainstem infarction: Confirmation of the benefit by anatomical characterization of false-negative lesions. 20-Sep-2019;10:180. Available from: http://surgicalneurologyint.com/surgicalint-articles/9663/
Abstract
Background: In some cases of acute brainstem infarction (BI), standard axial diffusion-weighted imaging (DWI) does not show a lesion, leading to false-negative (FN) diagnoses. It is important to recognize acute BI accurately and promptly to initiate therapy as soon as possible.
Methods: Of the 171 patients with acute cerebral infarctions in our institution who were examined, 16 were diagnosed with true-positive BI (TP-BI) and six with FN-BI. We evaluated the effectiveness of sagittal DWI in accurately diagnosing acute BI and sought to find the cause of its effectiveness by the anatomical characterization of FN-BIs.
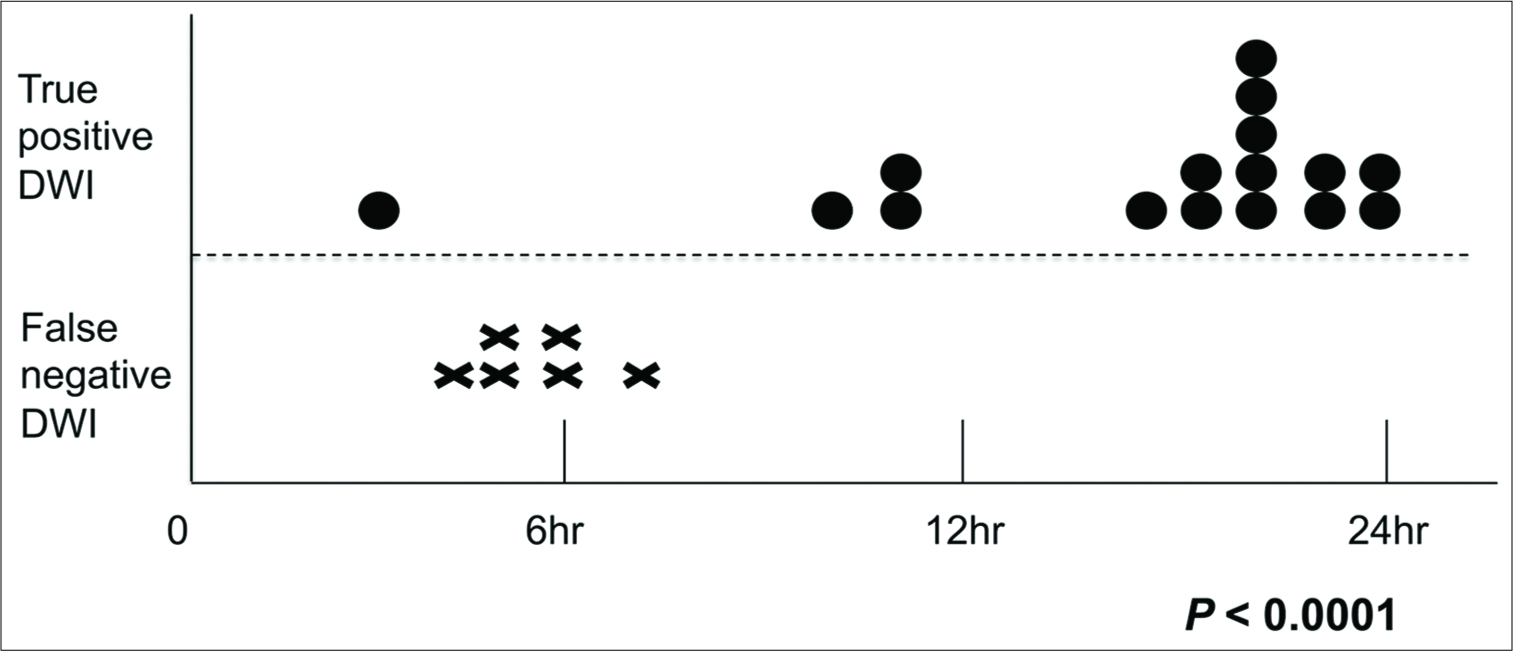
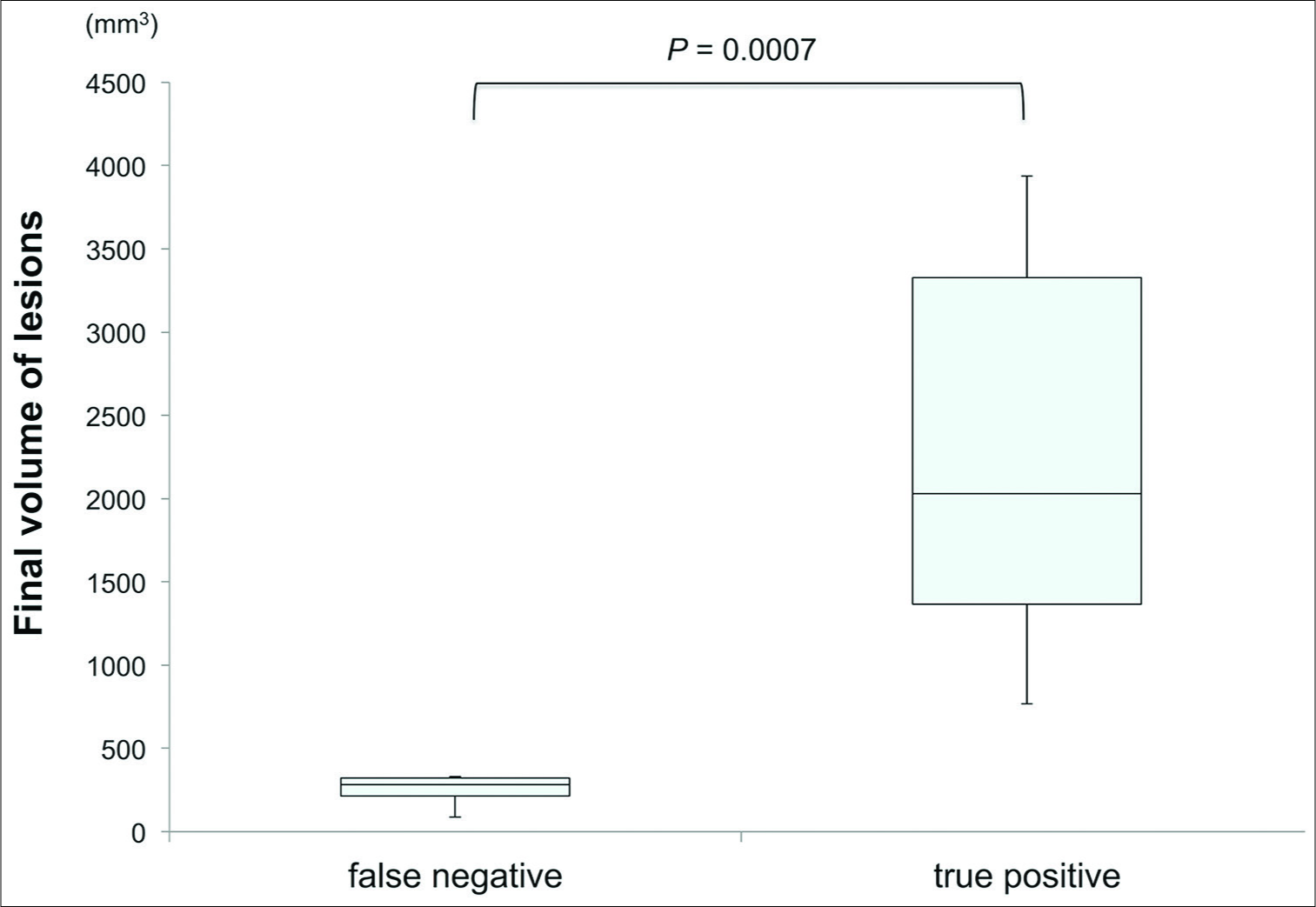
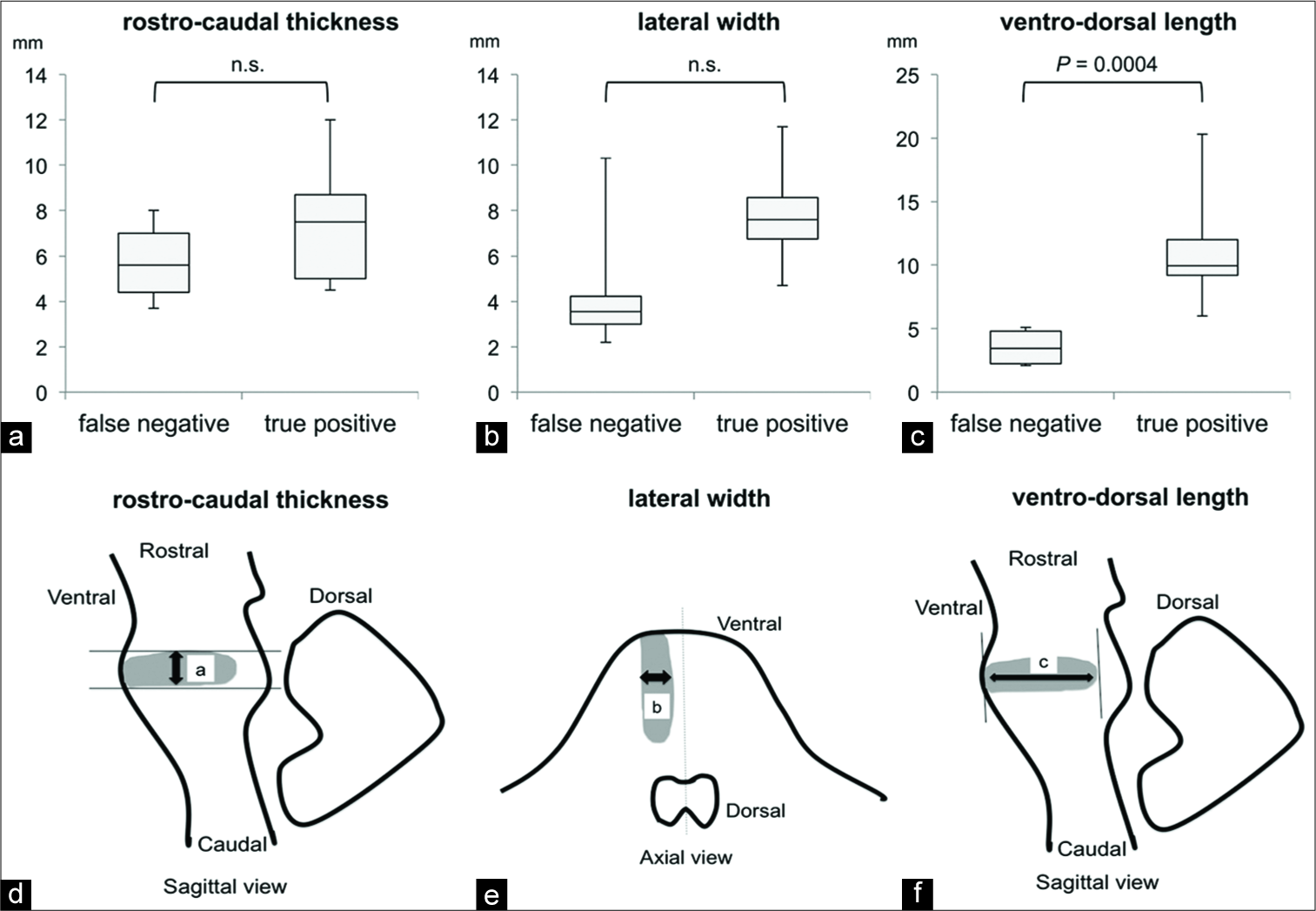
Results: Considering the direction of the brainstem perforating arteries, we supposed that sagittal DWI might more effectively detect BIs than axial DWI. We found that sagittal DWI detected all FN-BIs more clearly than axial DWI. The mean time between the onset of symptoms and initial DWI was significantly longer in the TP group (17.6 ± 5.5 h) than in the FN group (5.0 ± 1.2 h; P 3) than in TP-BIs (2779 ± 767 mm3; P = 0.0007). FN-BIs had a significant inverse correlation with the ventrodorsal length of infarcts (FN 3.5 ± 1.1 mm, TP 11.4 ± 3.6 mm; P
Conclusion: Anatomical characterization clearly confirmed that the addition of sagittal DWI to the initial axial DWI in suspected cases of BI ensures its accurate diagnosis and improves the patient’s prognosis.
Keywords: Brain stem infarctions, Diffusion-weighted magnetic resonance imaging, False negative
INTRODUCTION
Acute brainstem infarctions (BIs) account for about 10% of all acute ischemic strokes.[
As the anatomical direction of BI formation is along the paramedian arteries, we supposed that sagittal DWI would more effectively detect acute BI than axial and coronal DWI. Hence, we used sagittal DWI to accurately detect acute BIs in our study and found that all the BI lesions that were FN on the initial axial DWI were detected clearly on sagittal DWI. We also examined the features of FN-BIs and found that the inverse correlation between the FN lesions and the sagittal ventrodorsal length of the infarction was significant and might explain why sagittal DWI was more effective in detecting FN lesions than axial and coronal DWI. Thus, we recommend the use of sagittal DWI combined with standard axial DWI for making a more prompt and accurate diagnosis of acute BI, which might improve the patient’s prognosis, on the basis of anatomical characterization of FN-BIs.
MATERIALS AND METHODS
Subjects
This study was approved by the Institutional Review Board of Kurume University School of Medicine, Fukuoka, Japan. The procedures followed in this study were in accordance with the Helsinki Declaration of 1975, as revised in 2000. All authors had access to the data and statistical analysis and take responsibility for its integrity.
The clinical and radiological data of 171 consecutive patients with acute cerebral infarctions admitted in our hospital between January 2013 and December 2017, including data of 33 patients with BIs, were reviewed.
All patients with BIs who underwent DWI within 24 h of the onset of symptoms were included in this study, whereas patients whose final diagnoses were not infarction but included demyelinating diseases, central nervous system infections, and transient ischemic attacks were excluded from the study.
Before DWI examination, each patient was clinically evaluated by a neurologist and underwent computed tomography (CT) brain to rule out intracranial hemorrhage. In each case, the final diagnosis was made by the neurologist, who reviewed all the clinical and radiologic data available. DWI findings were assessed by two neurosurgeons and one neuroradiologist who were blinded to clinical data. The two neurosurgeons first examined each DWI and measured the size of the BI. Subsequently, as soon as possible, a neuroradiologist examined and confirmed their results and the BI size. In case of disparate opinions, the three doctors carefully reviewed the findings for the final results by observing and analyzing the DWI in detail. The diagnostic criteria of FN-BIs were as follows: (1) acute and clear brainstem symptoms and signs on arrival, even if subtle; (2) no high-intensity appearances on the initial axial DWI scan, with low apparent diffusion coefficient; and (3) definite diagnosis of BI on sagittal or coronal DWI performed just after the initial axial DWI scan.
Outcomes were presented as the scores with the modified Rankin scale (mRS) which is a simplified global assessment of function, in which a score of 0 indicates no impairment while a score of 5 indicates severe disability.[
Imaging
All patients underwent DWI examinations on a 3 Tesla Skyra scanner (Siemens, Germany). The standard axial DWI with single-shot echo-planar imaging covering the whole brain was initially performed with 5-mm thick slices and 1-mm gaps. Thin-sliced sagittal or coronal DWI only covered the width of the brainstem to decrease imaging time and was performed with 3-mm thick slices and 0.6-mm gaps each. The size of the ischemic lesion was measured on DWI by manually delineating the regions of interest. In each section, these areas were added and were multiplied with the slice thickness to determine the volume using the OsiriX (Pixmeo SARL, Bernex, Switzerland; Rosset, Spadola, and Ratib, 2004).
Statistical analysis
Statistical analyses were performed using the JMP® 13 (SAS Institute Inc., Cary, NC, USA). Results of all calculations are expressed as mean ± SD values. Descriptive data were analyzed using the Chi-square test or the Mann–Whitney U-test as appropriate. The lesion sizes on DWI were compared using the Mann–Whitney U-test. P < 0.05 was considered statistically significant.
RESULTS
Patients
Among the 171 patients with acute cerebral infarction who were included, 33 showed symptoms or signs suggestive of BI on admission. Five patients in whom DWI was not performed within 24 h of the onset of symptoms were excluded from the study. The remaining 28 patients were analyzed further. Six of them had etiologies other than BI: one had ischemia in other territories and one had nonischemic etiologies. Twenty-two patients (22/171: 13%) had BI which was confirmed as the final diagnosis. Among these 22 patients, 16 patients were diagnosed with BI using the initial axial DWI scan (true positive [TP]) and six patients were not (FN). Their lesions (FN lesions) were detected on sagittal or coronal DWI performed immediately after the axial DWI scan.
Thin-section sagittal DWI facilitating the accurate diagnosis of acute BI
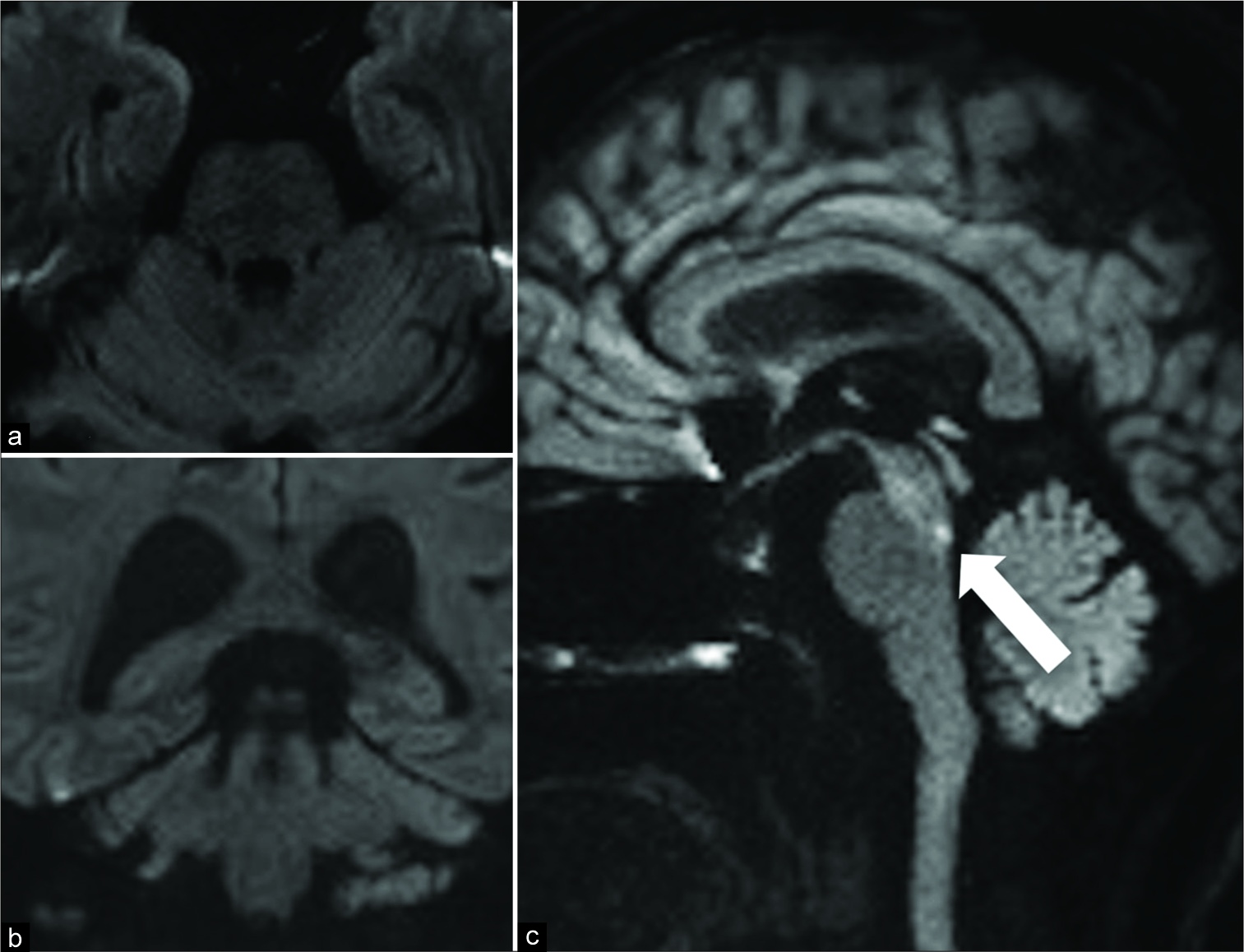
Figure 1:
The representative DWI of case 1 (an 85-year-old man with medial longitudinal fasciculus syndrome) that were taken 5 hours after the onset of symptoms is shown. No abnormal intensity is seen on a) standard axial, and b) coronal DWI scans; c) sagittal DWI scan shows a clear hyper-intensity, which is indicated by an arrow.
Six patients who were suspected to have BI on admission were consequently diagnosed as not having BI. Their sagittal DWI scan did not show positive signals in the brainstem, suggesting that sagittal DWI had a high negative predictive value for acute BI (6/6: 100%). This indicated that sagittal DWI had not only high sensitivity but also high specificity in diagnosing acute BI.
Characterization of false-negative DWI in acute BI
The FN rate of diagnosing BIs using axial DWI was 27.6% (6/22) while that of hemispheric infarctions was 1.2% (2/134) (P < 0.0001). The results were similar to those of the previous studies.[
The DWI lesions in the FN group were significantly smaller (final volume: 259 ± 82 mm3 [mean ± SD]) than those in the TP group (final volume: 2779 ± 767 mm3 [mean ± SD]) as determined by the Mann–Whitney U-test (P = 0.0007) [
Next, we compared the three-dimensional infarct size in the rostrocaudal, lateral, and ventrodorsal lengths [
DISCUSSION
The value of DWI in detecting acute BI is still controversial. Some studies report a high detection rate,[
Although the exact reason why FNs for acute BI occur remains unclear, several mechanisms have been suggested, such as the dense nuclei and fibers within the brainstem,[
There are a few previous reports on the clinical features of FN-BIs within 24 h of the onset of symptoms.[
We found that FN-BIs were more frequently diagnosed using standard axial DWI performed within 6 h of the onset of symptoms [
Furthermore, we also examined the effectiveness of coronal DWI in detecting BIs using three cases of FN-BIs [
The retrospective design and small number of participants limit our study. The reasons why the number of participants was small in this study were as follows. First, all patients with suspected BI on admission first underwent examination with CT scan to rule out hemorrhage and some patients subsequently underwent MRI examinations. We excluded the cases who underwent MRI examinations after 24 h of the onset of symptoms. Second, for the second MRI examination after the axial MRI, thin-sliced sagittal DWI was not performed for all cases.
CONCLUSION
Thin-section sagittal DWI combined with standard axial DWI was effective for the prompt and accurate diagnosis of BI in patients with suspected acute BI, thereby facilitating improved prognoses. The effectiveness was confirmed by the analyses comparing the three-dimensional infarct size in the ventrodorsal, lateral, and rostrocaudal lengths.
References
1. Alvarez-Linera J. Magnetic resonance techniques for the brainstem. Semin Ultrasound CT MR. 2010. 31: 230-45
2. Axer H, Grässel D, Brämer D, Fitzek S, Kaiser WA, Witte OW. Time course of diffusion imaging in acute brainstem infarcts. J Magn Reson Imaging. 2007. 26: 905-12
3. Ay H, Buonanno FS, Rordorf G, Schaefer PW, Schwamm LH, Wu O. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999. 52: 1784-92
4. Begemann M, Silvers A, Tang C, Tuhrim S. Delayed signal detection by diffusion-weighted imaging in brainstem infarction. J Stroke Cerebrovasc Dis. 2001. 10: 284-9
5. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014. 384: 1929-35
6. Engelter ST, Wetzel SG, Radue EW, Rausch M, Steck AJ, Lyrer PA. The clinical significance of diffusion-weighted MR imaging in infratentorial strokes. Neurology. 2004. 62: 574-80
7. Felfeli P, Wenz H, Al-Zghloul M, Groden C, Förster A. Combination of standard axial and thin-section coronal diffusion-weighted imaging facilitates the diagnosis of brainstem infarction. Brain Behav. 2017. 7: e00666-
8. Fitzek S, Fitzek C, Urban PP, Marx J, Hopf HC, Stoeter P. Time course of lesion development in patients with acute brain stem infarction and correlation with NIHSS score. Eur J Radiol. 2001. 39: 180-5
9. Frey LC, Sung GY, Tanabe J. Early false-negative diffusion-weighted imaging in brainstem infarction. J Stroke Cerebrovasc Dis. 2002. 11: 51-3
10. Krasnianski M, Lindner A, Zierz S. Brainstem infarctions with normal MRI. Eur J Med Res. 2002. 7: 125-7
11. Küker W, Weise J, Krapf H, Schmidt F, Friese S, Bähr M. MRI characteristics of acute and subacute brainstem and thalamic infarctions: Value of T2 and diffusion-weighted sequences. J Neurol. 2002. 249: 33-42
12. Kuruvilla A, Bhattacharya P, Rajamani K, Chaturvedi S. Factors associated with misdiagnosis of acute stroke in young adults. J Stroke Cerebrovasc Dis. 2011. 20: 523-7
13. Linfante I, Llinas RH, Schlaug G, Chaves C, Warach S, Caplan LR. Diffusion-weighted imaging and national institutes of health stroke scale in the acute phase of posterior-circulation stroke. Arch Neurol. 2001. 58: 621-8
14. Lövblad KO, Laubach HJ, Baird AE, Curtin F, Schlaug G, Edelman RR. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol. 1998. 19: 1061-6
15. Narisawa A, Shamoto H, Shimizu H, Tominaga T, Yoshimoto T. Diffusion-weighted magnetic resonance imaging (MRI) in acute brain stem infarction. No To Shinkei. 2001. 53: 1021-6
16. Oppenheim C, Stanescu R, Dormont D, Crozier S, Marro B, Samson Y. False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol. 2000. 21: 1434-40
17. Ortiz de Mendivil A, Alcalá-Galiano A, Ochoa M, Salvador E, Millán JM. Brainstem stroke: Anatomy, clinical and radiological findings. Semin Ultrasound CT MR. 2013. 34: 131-41
18. Querol-Pascual MR. Clinical approach to brainstem lesions. Semin Ultrasound CT MR. 2010. 31: 220-9
19. Schönfeld MH, Ritzel RM, Kemmling A, Ernst M, Fiehler J, Gellißen S. Improved detectability of acute and subacute brainstem infarctions by combining standard axial and thin-sliced sagittal DWI. PLoS One. 2018. 13: e0200092-
20. Sylaja PN, Coutts SB, Krol A, Hill MD, Demchuk AM, VISION Study Group. When to expect negative diffusion-weighted images in stroke and transient ischemic attack. Stroke. 2008. 39: 1898-900
21. Toi H, Uno M, Harada M, Yoneda K, Morita N, Matsubara S. Diagnosis of acute brain-stem infarcts using diffusion-weighed MRI. Neuroradiology. 2003. 45: 352-6
22. Tsuyusaki Y, Sakakibara R, Kishi M, Tateno F, Aiba Y, Ogata T. “Invisible” brain stem infarction at the first day. J Stroke Cerebrovasc Dis. 2014. 23: 1903-7
23. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988. 19: 604-7
24. Wardlaw JM, Armitage P, Dennis MS, Lewis S, Marshall I, Sellar R. The use of diffusion-weighted magnetic resonance imaging to identify infarctions in patients with minor strokes. J Stroke Cerebrovasc Dis. 2000. 9: 70-5
25. Wystub S, Laufs T, Schmidt M, Burmester T, Maas U, Saaler-Reinhardt S. Localization of neuroglobin protein in the mouse brain. Neurosci Lett. 2003. 346: 114-6