- Department of Neurosurgery, AZ Klina, Brasschaat, Belgium,
- Department of Radiology, AZ Klina, Brasschaat, Belgium,
- Department of Pulmonology, AZ Klina, Brasschaat, Belgium.
Correspondence Address:
Lars de Jong
Department of Neurosurgery, AZ Klina, Brasschaat, Belgium,
DOI:10.25259/SNI_51_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jasmien Rens1, Thomas Van Thielen2, Aurelie Derweduwen3, Koen Goedseels1, Robert Hes1, Lars de Jong1. Screening in cryptogenic brain abscess: Do not forget pulmonary arteriovenous malformations. 26-Apr-2021;12:188
How to cite this URL: Jasmien Rens1, Thomas Van Thielen2, Aurelie Derweduwen3, Koen Goedseels1, Robert Hes1, Lars de Jong1. Screening in cryptogenic brain abscess: Do not forget pulmonary arteriovenous malformations. 26-Apr-2021;12:188. Available from: https://surgicalneurologyint.com/surgicalint-articles/10755/
Abstract
Background: Brain abscess usually occurs secondary to trauma, through contiguous spread (e.g.; dental infections, [paranasal] sinusitis, otitis, and mastoiditis), after intracranial neurosurgical procedures, or through hematogenous spread in case of an arteriovenous (AV) shunt, for example; atrial septum defect. Although uncommon, another possible cause of AV shunt which can facilitate brain abscess is a pulmonary arteriovenous malformation (PAVM). We report a case of brain abscess secondary to a solitary PAVM and review the literature.
Case Description: A 74-year-old male patient presented with headaches, fatigue, low-grade fever, and homonymous hemianopsia. He was diagnosed with a brain abscess in the left occipital lobe. A chest computed tomography (CT) with intravenous (IV) contrast was performed because of fever and respiratory insufficiency in a period where screening for COVID-19 in suspected patients was important. A solitary PAVM of the left lung was diagnosed. Initial stereotactic burr hole drainage of the abscess was insufficient and resection of the abscess was deemed necessary. Routine workup did not reveal any additional pathology apart from the PAVM. After treatment of the cerebral abscess, the PAVM was treated with embolization using an endovascular plug.
Conclusion: It is recommended to screen for PAVM by chest CT with IV contrast in patients with brain abscess when no obvious source of infection can be identified.
Keywords: Brain abscess, Cryptogenic, Pulmonary arteriovenous malformation
INTRODUCTION
Brain abscess usually results from trauma, hematogenous spread through an atrial septal defect (ASD), contiguous spread from adjacent infections such as (paranasal) sinusitis or otitis media, or infections following intracranial neurosurgical procedures. In 20% of the cases, no source of infection can be identified and the abscess is deemed cryptogenic.[
CASE REPORT
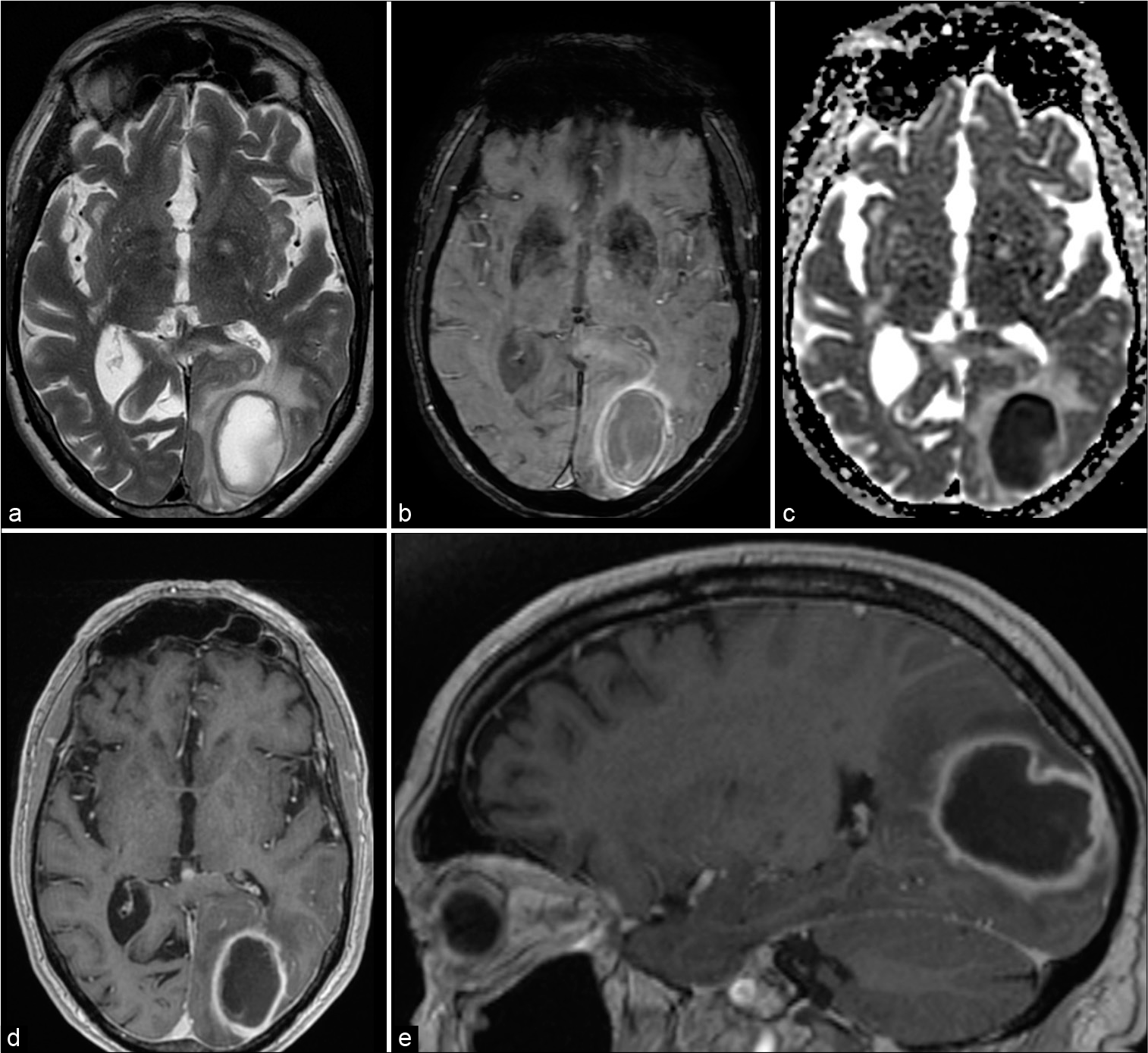
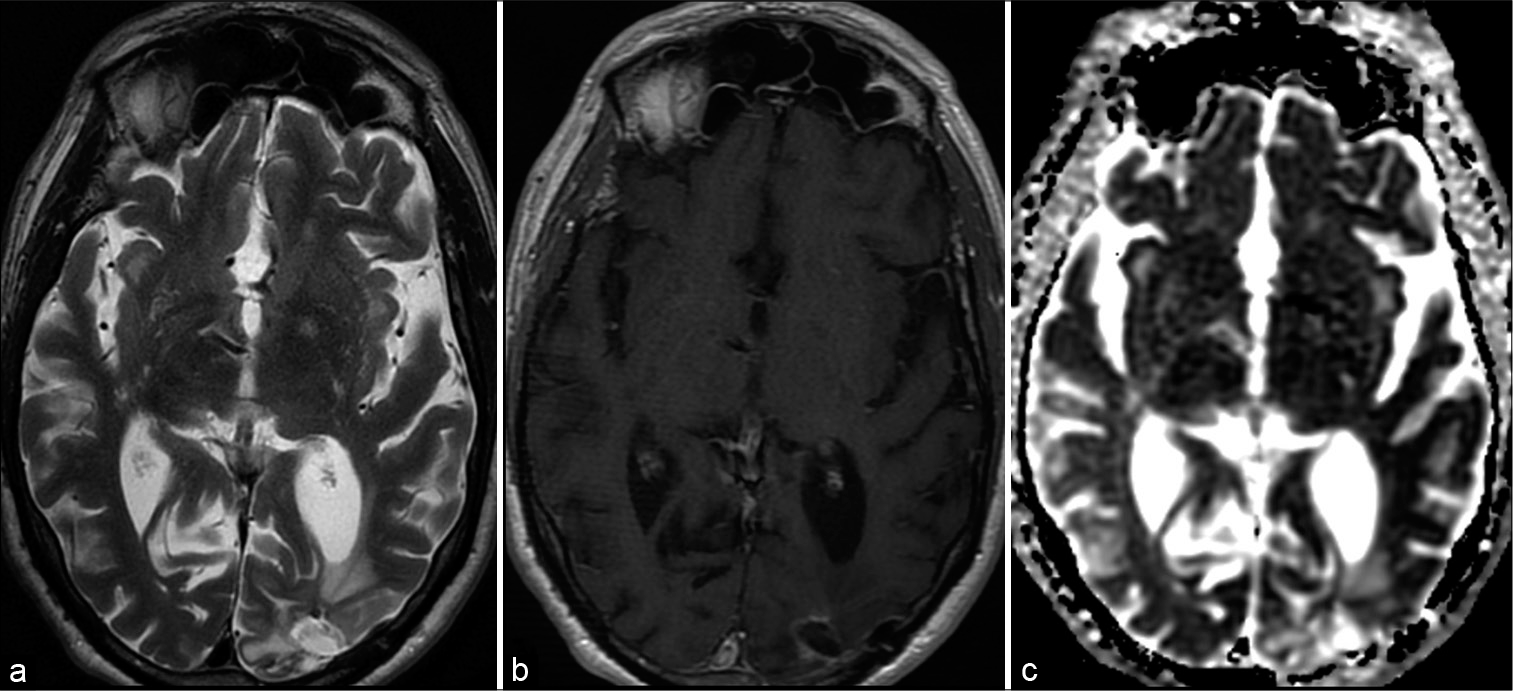
A 74-year-old male patient, without previous medical history, presented with headaches, fatigue, low-grade fever, disorientation, and difficulty conceiving distance. Neurological examination revealed a right-sided homonymous hemianopsia. During observation, body temperature fluctuated between 36.5 and 38°C. Oxygen saturation was 95%. Arterial blood samples showed a PaO2 of 67.9 mmHg, PaCO2 of 31.4 mmHg, and HCO3- of 22.1 mmol/L. Further, blood examination showed a slight raise in CRP (10.9 mg/L) but no leukocytosis. CT of the brain revealed a cystic lesion in the left occipital lobe with surrounding brain edema. Magnetic resonance imaging (MRI) confirmed this lesion, with central diffusion restriction suggestive for brain abscess [
Figure 1:
Magnetic resonance imaging shows typical brain abscess in the left occipital lobe. A T2 (a) hyperintense lesion with hypointense rim surrounded by vasogenic edema. Susceptibility-weighted images show a double rim sign (b), typical for brain abscess. Central diffusion restriction (ADC map – c). T1-weighted images (d and e) after gadolinium show rim enhancement.
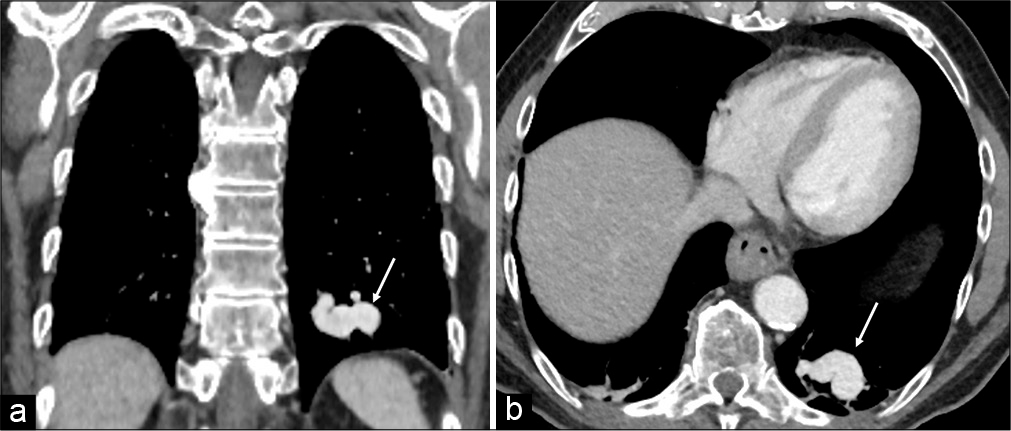
A contrast-enhanced CT of the chest, to exclude COVID-19 pneumonia, showed a solitary PAVM of the inferior lobe of the left lung [
Initial treatment consisted of stereotactic burr hole aspiration of 13 ml of purulent material and subsequent IV antibiotic therapy, consisting of ceftriaxone (2 g 2/d) and metronidazole (1.5 g 1/d). The antibiogram showed Streptococcus anginosus and Fusobacterium for which IV penicillin (4 million units 6/d) and metronidazole (1.5 g 1/d) were administered as definitive treatment. Despite a favorable biochemical evolution, the lesion progressively enlarged on MRI scan [
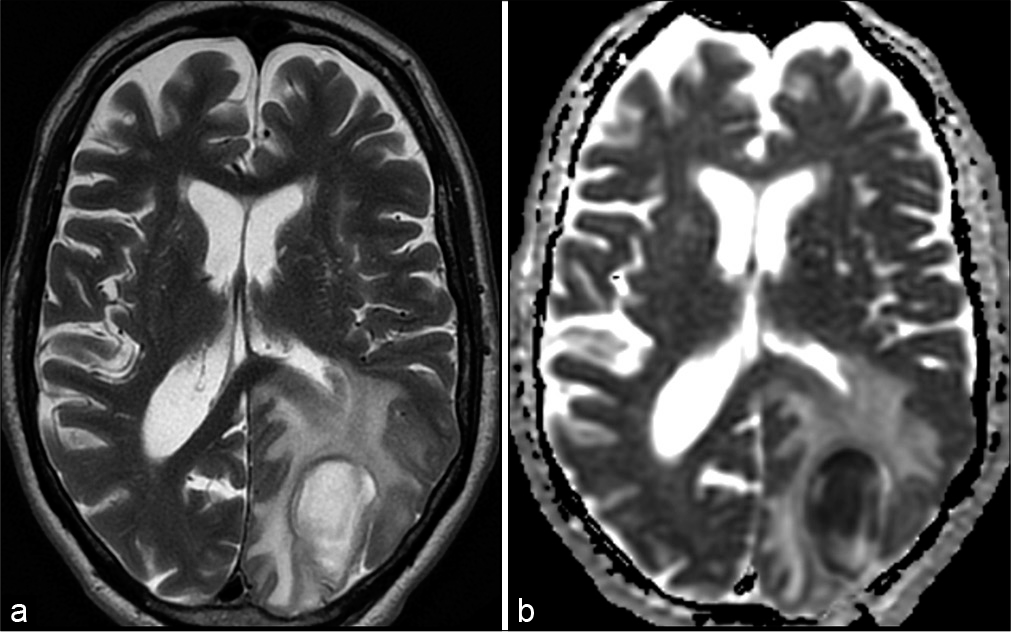
Figure 5:
Follow-up magnetic resonance imaging (5 months postoperatively) shows normal postoperative brain tissue loss and gliosis on T2-weighted image (a) in the left occipital lobe after abscess resection. On T1 (b) after gadolinium, there is no pathologic enhancement. There are no signs of diffusion restriction (ADC map – c).
LITERATURE REVIEW AND DISCUSSION
Brain abscess is a focal infection of the brain that can be caused by several microorganisms including bacteria, mycobacteria, protozoa, helminths, and fungi. Although present only in 20% of cases, the classic triad of symptoms includes headache, fever, and focal neurological deficits.[
Cranial imaging is crucial in the diagnosis of brain abscess and consists of CT scan with IV contrast enhancement and MRI with diffusion-weighted images to differentiate brain abscess from cystic or necrotic tumors.[
Brain abscess is often attributed to predisposing factors, such as disruption of the natural protective surrounding barriers (e.g.; neurosurgical procedure and trauma), an underlying disease, causing immunodeficiency, or a systemic source of infection. Bacteria can enter the brain through contiguous spread from infections of the middle or inner ear and sinuses or hematogenous spread from endocarditis, lung infections, or dental infections.[
When the diagnosis of brain abscess is made, a routine workup should be performed to find the source of infection. This includes echocardiography to rule out atrial septal defects, endocarditis, chest X-ray to identify lung infections, and evaluation of the teeth, ear, nose, and throat. [
PAVM has an estimated prevalence of 1 in 2600 people.[
In patients with cryptogenic brain abscess, we should look for clinical symptoms and signs of PAVM. Although often asymptomatic (66%), common symptoms are dyspnea, orthodeoxia, exercise intolerance, palpitations, and chest pain.[
Imaging is required for the diagnosis of PAVM. On chest radiographs, PAVM appears as a well-defined round or oval sharply defined nodule or mass. Chest radiographs are normal in 10–40% of cases, however, making routine screening insensitive.[
Optimal treatment of brain abscess includes both medical and surgical therapy. All patients should receive prompt empiric antibiotics such as ceftriaxone and metronidazole. The reason for choosing these drugs is their ability to penetrate in the abscess, and they cover both Gram-positive and -negative bacteria.[
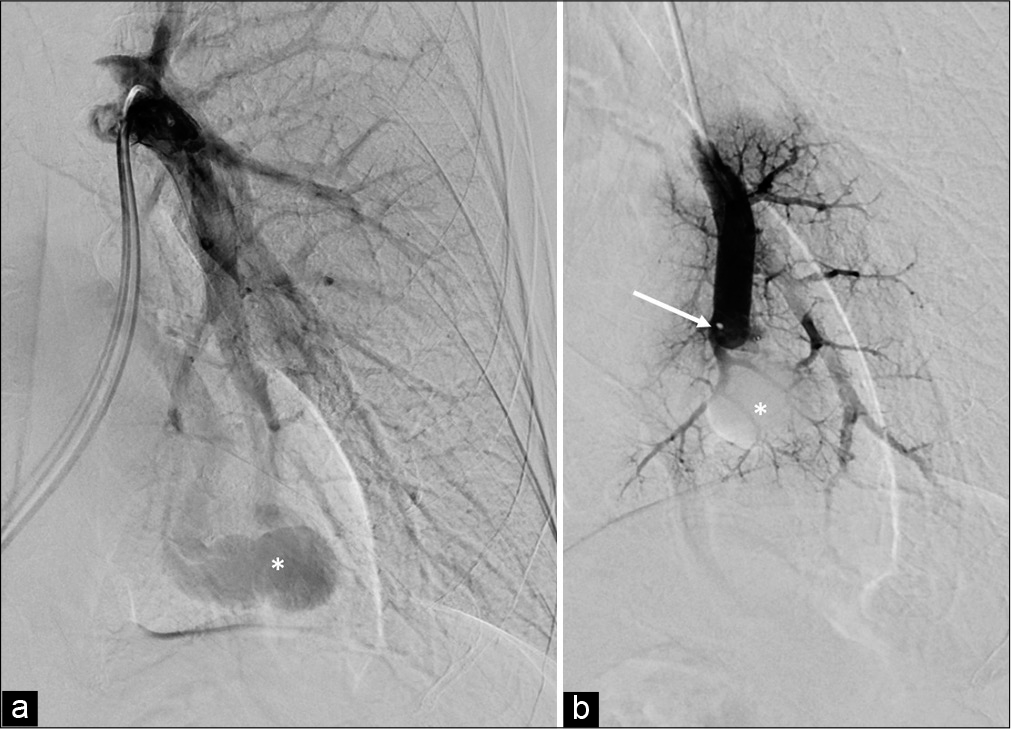
The current preferred treatment for PAVM is embolization, which is safe and effective in experienced hands. It is less invasive than surgical methods and repeatable.[
Although prognosis of brain abscess has improved with an all-cause mortality of 5–32%, many patients are unable to return to their former activities due to persistent neurological deficits.[
CONCLUSION
This case demonstrates the possibility of PAVM as a cause of brain abscess. Especially in cases of cryptogenic brain abscess, it is important to screen for PAVM. Treatment of PAVM can prevent recurrence of brain abscess. We recommend contrast-enhanced chest CT scan in all patients with cryptogenic brain abscess, particularly with evidence of right to left shunting on clinical examination or contrast echocardiography.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arivazhagan A, Pandey P, Anandh B, Abraham RG, Indira DB, Sampath S. An unusual etiology of recurrent cerebral abscesses-a report of 3 cases. Surg Neurol. 2009. 71: 241-4
2. Boother EJ, Brownlow S, Tighe HC, Bamford KB, Jackson JE, Shovlin CL. Cerebral abscess associated with odontogenic bacteremias, hypoxemia, and iron loading in immunocompetent patients with right-to-left shunting through pulmonary arteriovenous malformations. Clin Infect Dis. 2017. 65: 595-603
3. Brouwer MC, Tunkel AR, McKhann GM, van de Beek D. Brain abscess. N Engl J Med. 2014. 371: 447-56
4. Brouwer MC, van de Beek D. Epidemiology, diagnosis, and treatment of brain abscesses. Curr Opin Infect Dis. 2017. 30: 129-34
5. Cappa R, Du J, Carrera JF, Berthaud JV, Southerland AM. Ischemic stroke secondary to paradoxical embolism through a pulmonary arteriovenous malformation: Case report and review of the literature. J Stroke Cerebrovasc Dis. 2018. 27: e125-e127
6. Cartin-Ceba R, Swanson KL, Krowka MJ. Pulmonary arteriovenous malformations. Chest. 2013. 144: 1033-44
7. Cox M, Patel M, Deshmukh S, Roth CG, Flanders AE. Contrast-enhanced chest computed tomography reveals treatable causes of cerebral abscesses in patients without antecedent surgery or trauma. World Neurosurg. 2017. 101: 144-8
8. Dong SL, Reynolds SF, Steiner IP. Brain abscess in patients with hereditary hemorrhagic telangiectasia: Case report and literature review. J Emerg Med. 2001. 20: 247-51
9. Holzer RJ, Cua CL. Pulmonary arteriovenous malformations and risk of stroke. Cardiol Clin. 2016. 34: 241-6
10. Ibrahim I, Rabiou S, Laila B, Zahra AF, Jamal G, Marouane L. Cerebral abscesses revealing pulmonary arteriovenous malformations. Chin Med J (Engl). 2016. 129: 2253-5
11. Kaido T, Moriyama Y, Ueda K, Higashiura W, Sakaguchi H, Kichikawa K. Recurrent brain abscess induced by pulmonary arteriovenous fistula. J Infect Chemother. 2011. 17: 552-4
12. Kroon S, Snijder RJ, Faughnan ME, Mager HJ. Systematic screening in hereditary hemorrhagic telangiectasia: A review. Curr Opin Pulm Med. 2018. 24: 260-8
13. Lacombe P, Lacout A, Marcy PY, Binsse S, Sellier J, Bensalah M. Diagnosis and treatment of pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: An overview. Diagn Interv Imaging. 2013. 94: 835-48
14. Patel K, Clifford DB. Bacterial brain abscess. Neurohospitalist. 2014. 4: 196-204
15. Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography. 1993. 10: 397-403
16. Shovlin CL, Condliffe R, Donaldson JW, Kiely DG, Wort SJ. British thoracic society clinical statement on pulmonary arteriovenous malformations. Thorax. 2017. 72: 1154-63
17. Shovlin CL, Jackson JE, Bamford KB, Jenkins IH, Benjamin AR, Ramadan H. Primary determinants of ischaemic stroke/ brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax. 2008. 63: 259-66
18. Shovlin CL. Pulmonary arteriovenous malformations. Am J Respir Crit Care Med. 2014. 190: 1217-28
19. Tabakow P, Jarmundowicz W, Czapiga B, Czapiga E. Brain abscess as the first clinical manifestation of multiple pulmonary arteriovenous malformations in a patient with hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease). Folia Neuropathol. 2005. 43: 41-4