- Department of Neurosurgery, Nagahama City Hospital, Nagahama, Shiga, Japan.
- Department of Cardiology, Nagahama City Hospital, Nagahama, Shiga, Japan.
DOI:10.25259/SNI_244_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masako Fujii1, Takeshi Satow1, Hiroshi Kodama2, Satoshi Horiguchi1. Severe hemodynamic depression after carotid artery stenting: The problem overcome with a transvenous temporary cardiac pacemaker. 07-Jun-2021;12:255
How to cite this URL: Masako Fujii1, Takeshi Satow1, Hiroshi Kodama2, Satoshi Horiguchi1. Severe hemodynamic depression after carotid artery stenting: The problem overcome with a transvenous temporary cardiac pacemaker. 07-Jun-2021;12:255. Available from: https://surgicalneurologyint.com/surgicalint-articles/10872/
Abstract
Background: Carotid angioplasty stenting (CAS) may have adverse events including perioperative hemodynamic depression. A transvenous temporary cardiac pacemaker (TTCP) is an option for preventing devastating sequelae due to circulatory failure. An exploration of the predictors of hemodynamic depression following CAS is valuable for selecting candidates for preoperative TTCP implantation before CAS.
Case Description: An 84-year-old man underwent CAS for asymptomatic left carotid severe stenosis. He had no history of bradycardia arrhythmia. A TTCP was implanted in advance in view of the likelihood of perioperative hemodynamic depression. CAS was accomplished successfully, but severe hypotension and vanishing of self-heartbeat occurred about 90 min after the procedure. By activating the pre-implanted TTCP, spontaneous circulation was readily recovered with vasopressor administration. He was discharged with no additional neurological deficits. A literature review using a random effect model found that smoking (odds ratio [OR] 1.68, 95% confidence interval (CI) 1.13–2.52) and severely calcified plaque (OR 3.70, 95% CI 2.15–6.35) were significant predictors of perioperative hemodynamic depression following CAS.
Conclusion: TTCP can be recommended for a patient receiving CAS to prevent catastrophic consequences, particularly in cases with a history of smoking or severely calcified plaque.
Keywords: Bradycardia, Carotid artery stenting, Hypotension, Predictor, Temporary cardiac pacemaker
INTRODUCTION
Carotid artery stenosis is one of the causes of ischemic stroke, in which surgery is a treatment option for selected cases. Carotid endarterectomy (CEA) is the standard long-standing surgical procedure and was proved to be effective in preventing ischemic stroke in patients with moderate to severe carotid stenosis as long as three decades ago.[
Apart from perioperative ischemic complications, CAS often involves hemodynamic instabilities such as hypotension and bradycardia, at least during the period of balloon inflation. A recent study found that periprocedural hemodynamic instability predicted periprocedural stroke, myocardial infarction, death, and longer hospitalization.[
We report here a patient in whom severe circulatory depression after CAS was readily managed as a result of the previously implanted TTCP, and we discuss the necessity of TCP in CAS. We have also conducted a meta-analysis of the literature concerning predictors of periprocedural hemodynamic depression following CAS.
CASE REPORT AND INTERVENTION
An 84-year-old man, with a history of hypertension and chronic kidney disease, was referred to the emergency room with a complaint of dysphagia and hand clumsiness on his left side since the previous day. He had no history of smoking and alcohol drinking. The National Institutes of Health Stroke Scale score was 2. Magnetic resonance imaging (MRI) of the brain revealed an acute infarction in the right frontal insuloopercular area [
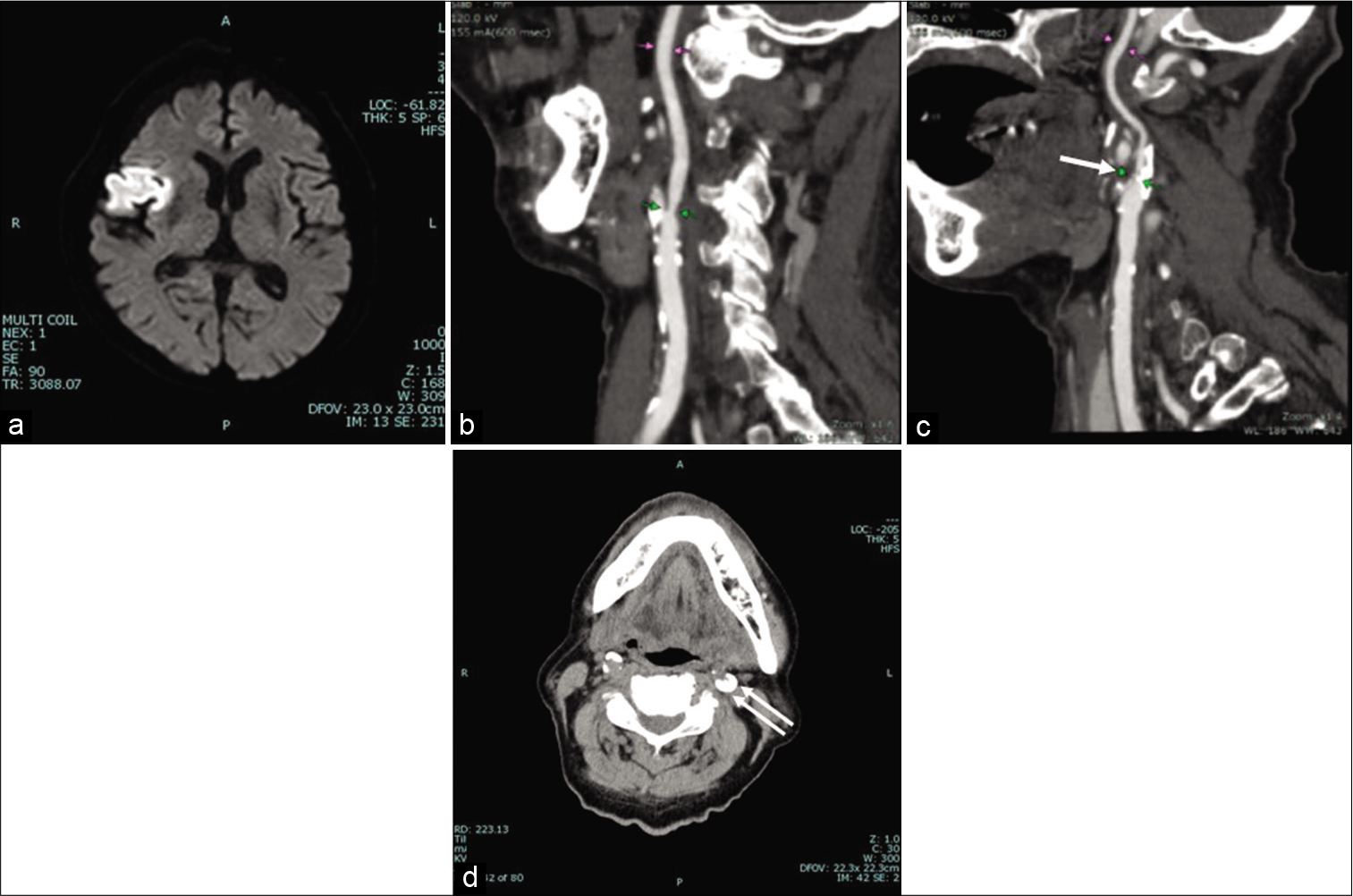
Figure 1:
(a) Diffusion-weighted image at initial presentation showing the right frontal insulo-opercular infarction. Sagittal image of CT angiogram of (b) the right and (c) the left carotid artery, showing mild stenosis on the right and severe stenosis on the left side (arrow). (d) CT without contrast medium of the neck, showing severe calcified carotid plaque on the left side (double arrows).
An electrocardiogram (ECG) found a normal sinus rhythm. The man had no history of atrial fibrillation (AF) or bradycardia. ECG monitoring did not capture any episode of AF. His baseline serum low-density lipoprotein cholesterol level was 59 mg/dl, and his high-density lipoprotein cholesterol level was 121 mg/dl.
A computed tomography (CT) angiogram found bilateral carotid artery stenosis, which was more severe on the left side (right: 47%, left: more than 90%, on NASCET criteria[
Since the symptomatic right side demonstrated stenosis <50%, we proposed to manage the right IC stenosis conservatively. For the asymptomatic side, showing severe stenosis, carotid angioplasty was planned to prevent a further ischemic stroke.
After obtaining written informed consent from the patient and his family, we performed CAS for the left high-grade carotid stenosis, because of preexisting dysphagia.
TTCP
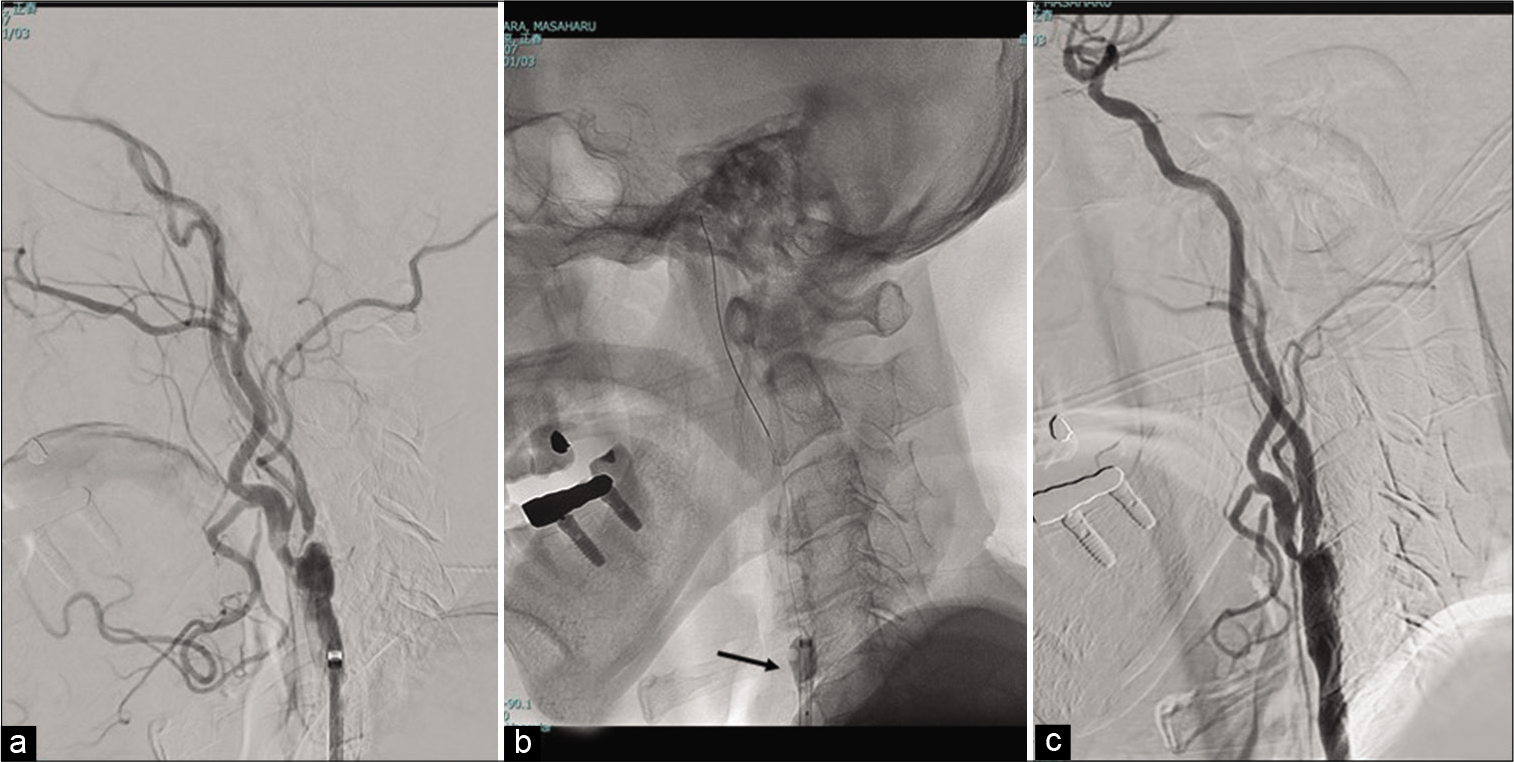
Before CAS, the transvenous cardiac pacemaker was placed through the left femoral vein, because the serious hemodynamic depression was expected due to severe calcified plaque [
Surgical procedure
Under intravenous sedation with propofol, and local infiltration anesthesia with lidocaine, a 9-Fr occlusion balloon-guiding catheter (OPTIMO; Tokai Medical Products, Aichi, Japan) was inserted through the right common femoral artery, and was placed at the left common carotid artery. Unfractionated heparin (100 units/kg of body weight) was given intravenously to keep the activated clotting time above 300 s. The selective digital subtraction angiogram showed a severe stenosis of the left carotid artery [
Because of the difficulty in navigating a filter-based embolic protection device, we carried out the procedure with proximal balloon protection [
After the intravenous injection of atropine (0.5 mg), a balloon catheter of small diameter (UNRYU 2 mm, Kaneka, Japan) was induced to the stenotic region with a micro-guidewire (Chikai 0.014, ASAHI INTECC, Japan) and was inflated at 6 atm for 30 s. Predilation was then performed using a 4 mm balloon catheter (RX-Genity, Kaneka, Japan). A self-expandable open-cell stent (4 × 40 mm, PRECISE Pro RX, Cordis Japan) was deployed successfully, with no post dilation [
There was no bradycardia during the surgical procedure. Blood pressure was decreased depending on the propofol infusion, which was managed by means of intermittent administration of 1 mg etilefrine [
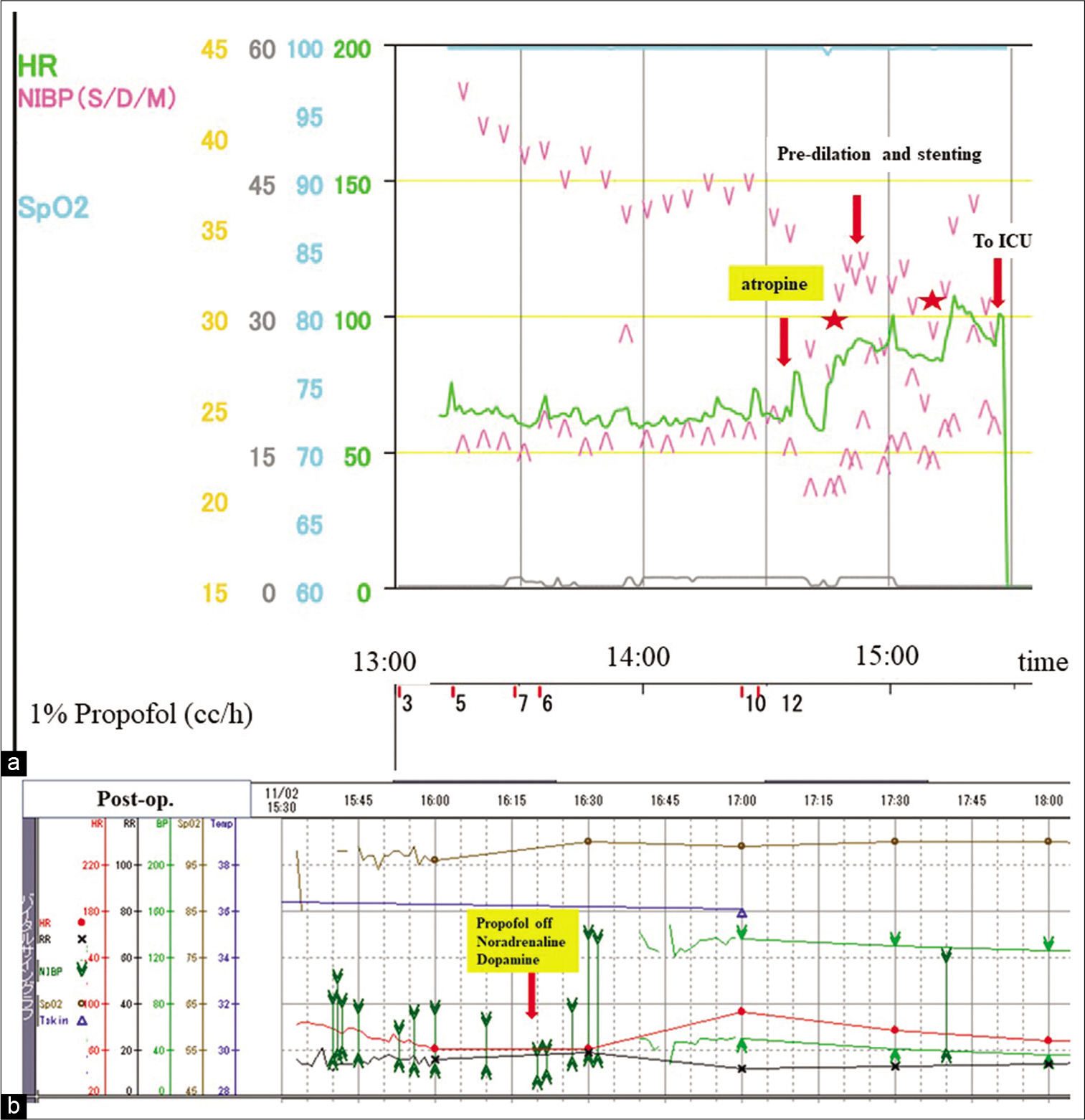
Figure 3:
(a) Intraoperative and (b) postoperative cardiopulmonary monitoring chart. (a) For sedation, propofol was administered continuously, causing a gradual decrease in systolic blood pressure (sBP). Atropine was given intravenously, and increases in sBP and heart rate (HR) were confirmed. Balloon angioplasty with stent deployment was then conducted successfully. (b) About 45 min after ICU admission, severe circulatory depression developed, but recovery from it was accomplished immediately on cessation of propofol and administration of noradrenaline and dopamine. HR: Heart rate, NIBP: Non-invasive blood pressure, S: Systolic, D: Diastolic, M: Mean, SpO2: Percutaneous oxygen saturation, ICU: Intensive care unit.
Postoperative course
Blood pressure gradually decreased, necessitating a reduction in the infusion of Propofol. Approximately 45 min after transfer to the intensive care unit (about 90 min after the procedure), blood pressure suddenly fell to 37/18, and his heart beat depended completely on TTCP [
Postoperative MRI did not show any high-intensity signal on a diffusion weighted image. Aspiration pneumonia associated with acute heart failure was observed on postoperative day (POD) 5; this was managed with antibiotics, diuretics and high flow nasal cannula oxygen therapy. SPECT on POD4 revealed hyperperfusion in the left middle cerebral artery area without any corresponding symptoms, which subsequently subsided POD11.
After confirmation of absence of re-stenosis or plaque protrusion, using USG, the patient was discharged home on POD 26 without any neurological sequelae on POD 26.
Predictors of periprocedural hemodynamic depression after CAS by literature review
Two authors (M.F. and T.S.) independently searched the PubMed database up to the last day of 2020 with the following keywords: (“bradycardia” OR “hypotension” OR “hemodynamic instability”) AND (“carotid” AND (“artery” OR “angioplasty”) AND (“stent” OR “stenting”) OR (“carotid” AND “stenosis”) AND (“predictor” AND “risk factor”). This initial literature search yielded 23 articles.
Studies eligible for inclusion had the following features: (1) case series observational studies, whether retrospective or prospective, in which at least 100 CAS procedures were conducted and the predictors of periprocedural hemodynamic depression were investigated (hypotension and /or bradycardia); (2) the stent used was explicitly described as self-expandable; (3) raw data of predictors of interest could be extracted adequately and (4) age, mean and standard deviation were presented; and (5) the articles were peer-reviewed and written in English. Studies in which the specific CAS technique was investigated in relation to periprocedural hemodynamic instability were excluded from analysis.
From each article, we extracted data for the following parameters: age, sex (male), hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, peripheral artery disease, chronic obstructive pulmonary disease, smoking, stenosis with severe calcification, lesion near the carotid bulb (within 10 mm), side of the lesion (right), and symptomatic lesion. Any discordant results were resolved by discussion. The odds ratio (OR) and 95% confidence intervals (CIs) for periprocedural hemodynamic depression were then computed through the Mantel-Haenszel meta-analytic method. For age, the mean difference and 95% CIs were calculated using the inverse variance method. Statistical analysis was performed using R version 4.0.3 (R Core Team) and free software EZR.[
The inclusion and exclusion criteria specified above gave us four articles,[
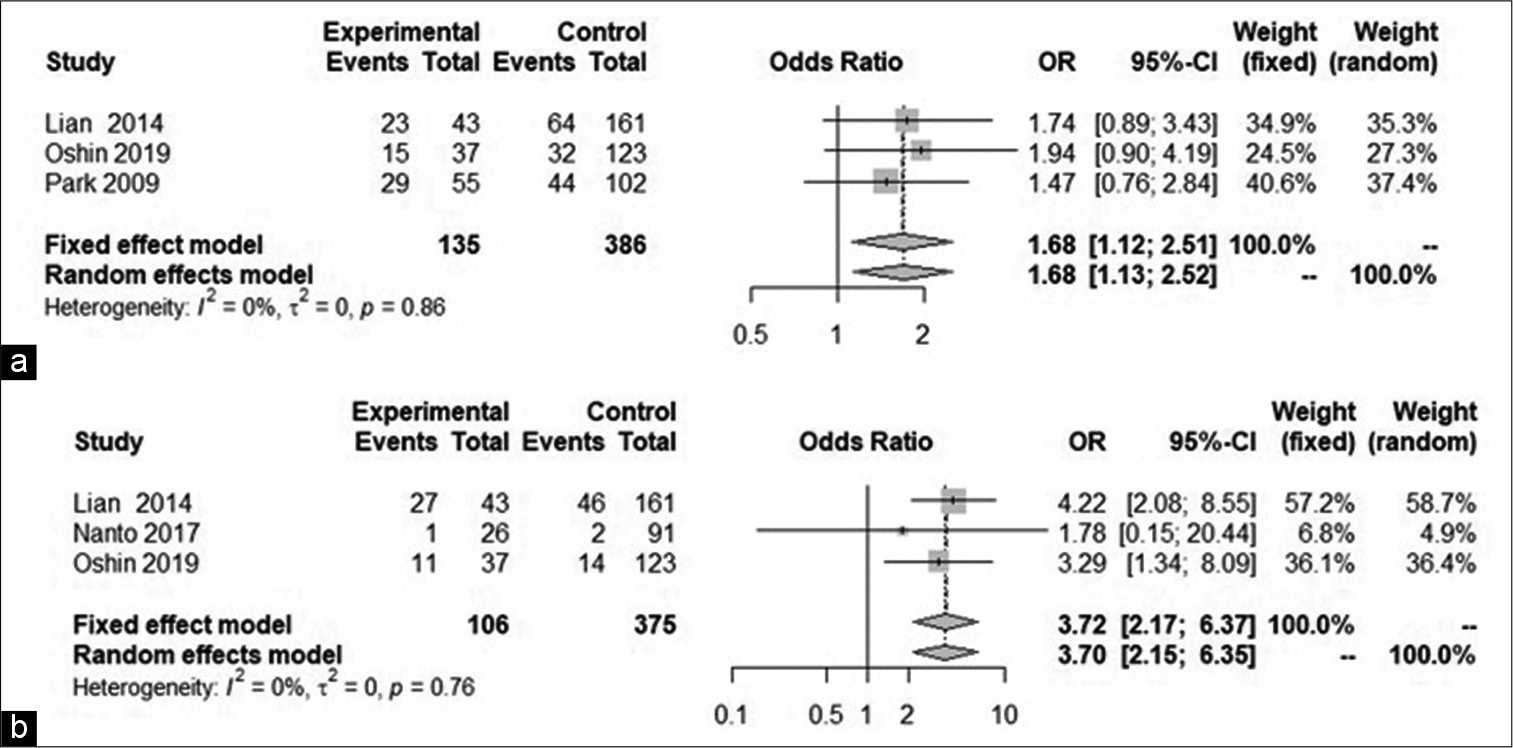
Based on our random effects meta-analysis model for all variables, significant predictors of periprocedural hemodynamic depression were found to be “smoking” (OR 1.68, 95% CI 1.13–2.52, P = 0.01) and “calcification” (OR 3.70, 95% CI 2.15–6.35, P < 0.0001); [
DISCUSSION
In the present case, severe circulatory depression following CAS was managed relatively easily due to the preexisting TTCP. If this TTCP had not been implanted, the measures for acute circulatory deterioration would have been more complicated, possibly resulting in a fatal outcome.
Circulatory instability is frequently seen after CAS occurring immediately (1 h) or within 20 h at latest;[
The value of preoperative TTCP for CAS has been reported previously,[
Based on our meta-analysis of the literature, “smoking” and “severe calcification” were significant predictors of preprocedural hemodynamic depression. We, therefore, recommend, particularly in patients with severely calcified plaque and a history of smoking, that TTCP be implemented before CAS, given informed consent.
CONCLUSION
TTCP should be considered in performing CAS to prevent and promptly treat the resulting hemodynamic depression, particularly in patients with severely calcified plaque and a history of smoking.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank SCITEXT Cambridge for English language editing.
References
1. Arhuidese IJ, Ottinger ME, Shukla AJ, Moudgil N, Armstrong P, Illig K. Hemodynamic events during carotid stenting are associated with significant periprocedural stroke and adverse events. J Vasc Surg. 2020. 71: 1941-53.e1
2. Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010. 363: 11-23
3. Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016. 374: 1021-31
4. Bush RL, Lin PH, Bianco CC, Hurt JE, Lawhorn TI, Lumsden AB. Reevaluation of temporary transvenous cardiac pacemaker usage during carotid angioplasty and stenting: A safe and valuable adjunct. Vasc Endovascular Surg. 2004. 38: 229-35
5. Carrizales-Sepúlveda EF, González-Sariñana LI, Ordaz-Farías A, Vera-Pineda R, Flores-Ramírez R. Thermal burn resulting from prolonged transcutaneous pacing in a patient with complete heart block. Am J Emerg Med. 2018. 36: 1523.e5-6
6. D’Arrigo S, Cacciola S, Dennis M, Jung C, Kagawa E, Antoenelli M. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: A systematic review and meta-analysis. Resuscitation. 2017. 121: 62-70
7. 8. Gupta R, Horowitz M, Jovin TG. Hemodynamic instability after carotid angioplasty and stent placement: A review of the literature. Neurosurg Focus. 2005. 18: E6 9. Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomized controlled trial. Lancet. 2004. 363: 1491-502 10. Harrop JS, Sharan AD, Benitez RP, Armonda R, Thomas J, Rosenwasser RH. Prevention of carotid angioplasty-induced bradycardia and hypotension with temporary venous pacemaker. Neurosurgery. 2001. 49: 814-22 11. Im SH, Han MH, Kim SH, Kwon BJ. Transcutaneous temporary cardiac pacing in carotid stenting: Noninvasive prevention of angioplasty-induced bradycardia and hypotension. J Endovasc Ther. 2008. 15: 110-6 12. Kanda Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statics. Bone Marrow Transplant. 2013. 48: 452-8 13. Lian X, Lin M, Liu M, Huang J, He X. Complications and predictors associated with persistent hemodynamic depression after carotid artery stenting. Clin Neurol Neurosurg. 2014. 124: 81-4 14. Liu J, Yao GE, Zhou HD, Jiang XJ, Chen Q. Prevention of hemodynamic instability in extra-cranial carotid angioplasty and stenting using temporary transvenous cardiac pacemaker. Cell Biochem Biophys. 2013. 65: 275-9 15. McKevitt FM, Sivaguru A, Venables GS, Cleveland TJ, Gaines PA, Beard JD. Effect of treatment of carotid artery stenosis on blood pressure. A comparison of hemodynamic disturbances after carotid endarterectomy and endovascular treatment. Stroke. 2003. 34: 2576-82 16. Nanto M, Goto Y, Yamamoto H, Tanigawa S, Takeuchi H, Nakahara Y. Complications and predictors of hypotension requiring vasopressor after carotid artery stenting. Neurol Med Chir (Tokyo). 2017. 57: 115-21 17. Barnett HJ, Taylor DW, Haynes RB, Sackett DL, Peerless SJ. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991. 325: 445-53 18. Oshin O, Varcoe R, Wong J, Burrows S, Altaf N, Schlaich M. Multivariable analysis of patients with severe persistent postprocedural hypotension after carotid artery stenting. J Endovasc Ther. 2019. 26: 759-67 19. Park BD, Divinagracia T, Madej O, McPhelimy C, Piccirillo B, Dahn MS. Predictors of clinically significant postprocedural hypotension after carotid endarterectomy and carotid angioplasty with stenting. J Vasc Surg. 2009. 50: 526-33 20. Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med. 2016. 374: 1011-20 21. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004. 351: 1493-501