- McGill University Health Centre, Montreal, Quebec, Canada
- Department of Neurosurgery, Kochi University, Kochi
- Faculty of Medicine, The University of Tokyo, Tokyo
- Department of Surgery, Division of Neurosurgery, Queen Mary Hospital, University of Hong Kong, Hong Kong
- Amagasaki General Medical Center, Kyoto University, Hyogo, Japan
DOI:10.25259/SNI-74-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Y. Lo BW, Fukuda H, Miyawaki S, O. Tsang AC, Koyanagi M. Short review of randomized controlled trials for Surgical Neurology International: Part II - drain-associated cerebrospinal fluid infections. Surg Neurol Int 26-Mar-2019;10:39
How to cite this URL: Y. Lo BW, Fukuda H, Miyawaki S, O. Tsang AC, Koyanagi M. Short review of randomized controlled trials for Surgical Neurology International: Part II - drain-associated cerebrospinal fluid infections. Surg Neurol Int 26-Mar-2019;10:39. Available from: http://surgicalneurologyint.com/surgicalint-articles/9245/
INTRODUCTION
Two methodologically sound randomized controlled trials (RCTs) were published in the third and fourth quarters of 2018 dealing with drain-associated cerebrospinal fluid (CSF) infections. Both RCTs offer significant scientific findings which may be of interest to readers of Surgical Neurology International.
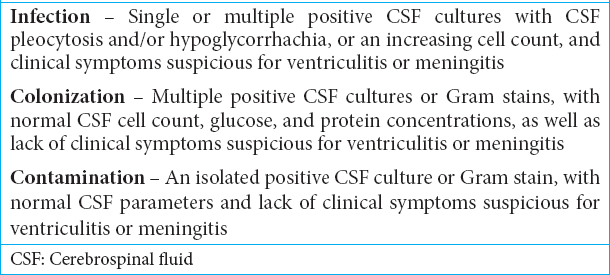
Infectious diseases society of America’s 2017 clinical infectious disease guidelines for external ventricular drains
Drain-associated CSF infections have a pooled incidence rate of about 11.4/1000 catheter days.[
Infections due to external ventricular drains and lumbar drains
Most infections related to external ventricular or lumbar drains are introduced at the time of drain placement, whereas retrograde infection is another, very secondary mechanism of contamination. Risk factors for ventriculostomy-associated CSF infections include (1) previous craniotomy, (2) systemic infection, (3) skull fracture with CSF leak, (4) intraventricular/subarachnoid hemorrhage, (5) increased duration of drain, and (6) procedural factors (e.g., drain site leaks, lack of tunneling, multiple catheter irrigations, and increased frequency of CSF sampling).[
Use and efficacy of prophylactic antibiotics
Prophylactic antimicrobial agents have not been shown to significantly reduce drain-associated CSF infections in patients without significant risk factors.[
The SiLuDrain trial
The SiLuDrain trial is a single-center RCT from Germany comparing infection and complication rates utilizing conventional versus silver-impregnated lumbar drains (both of 1.6-mm outer diameter and 0.8-mm inner diameter).[
Lumbar drain protocol
All patients had lumbar drains placed for an average of 4 days (range 1–10 days). Daily inspection of puncture sites was accompanied by routine CSF sampling on the day of drain placement and every 2nd day until drains were removed. Here, drain-associated CSF infection was defined as a confirmed cultured organism or clinical sign suggestive of meningitis. Patients in the silver-impregnated lumbar drain group experienced significantly fewer catheter-related complications [
The external ventricular drain-associated infection (EVDAI) study
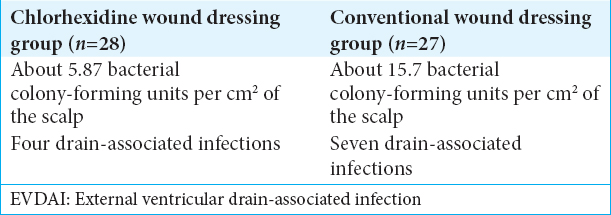
In Roethlisberger et al., single-center controlled RCT investigators studied scalp bacterial growth and silver-impregnated ventriculostomy colonization in patients with and without chlorhexidine wound dressings (chlorhexidine gluconate 2%).[
Culture from the scalp
Sampling of scalp at drain exit sites was performed at the time of procedure for a skin area of 12 cm2, with dressing changes every 5 days. Those with silver-impregnated ventriculostomies placed for an average of 8 days (range 5–12 days), utilizing the chlorhexidine protocol, exhibited significantly less bacterial growth versus those with uncoated dressings [
DISCUSSION
The SiLuDrain trial and EVDAI study offer insight regarding the prevention of drain-associated infections. The SiLuDrain trial demonstrated that silver-impregnated lumbar drains are well tolerated with fewer Gram-positive bacterial infections. Findings from this study are consistent with prior meta-analysis of prospective studies demonstrating the effectiveness of silver-coated ventricular drains in preventing ventriculostomy-associated infections.[
Most drain-associated infections are caused by bacteria introduced at the time of drain insertion and subsequent catheter colonization, possibly as a result of scalp bacterial growth. The EVDAI study demonstrated reduced scalp bacterial growth, including fewer virulent Gram-positive strains, with the use of chlorhexidine wound dressings.[
Protocols for drain insertion and care can raise awareness of drain-associated CSF infection risk factors. Management of these risk factors, including multiple drain exchanges, frequent CSF samplings, and catheter duration, is essential to decrease patient morbidity and health-care-related costs associated with CSF infections and long-term likelihood of requiring permanent CSF diversion.
CONCLUSIONS
Two 2018 RCTs demonstrated that silver-coated drains were effective in decreasing Gram-positive bacterial adherence to catheter tips and subsequent colonization. They may not be as effective as antibiotic-impregnated drains in preventing Gram-negative bacterial colonization. Furthermore, antimicrobial-coated chlorhexidine dressings appear to be effective in suppressing scalp bacterial growth.
Disclaimer
The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Journal or its management.
References
1. Atkinson RA, Fikrey L, Vail A, Patel HC. Silver-impregnated external-ventricular-drain-related cerebrospinal fluid infections: A meta-analysis. J Hosp Infect. 2016. 92: 263-72
2. Atkinson R, Fikrey L, Jones A, Pringle C, Patel HC. Cerebrospinal fluid infection associated with silver-impregnated external ventricular drain catheters. World Neurosurg. 2016. 89: 505-9
3. Jakobs M, Klein S, Eigenbrod T, Unterberg AW, Sakowitz OW. The siLuDrain trial: A prospective randomized controlled trial comparing standard versus silver-impregnated lumbar drains. J Neurosurg. 2018. p. 1-8
4. Keong NC, Bulters DO, Richards HK, Farrington M, Sparrow OC, Pickard JD. The SILVER (Silver impregnated line versus EVD randomized trial): A double-blind, prospective, randomized, controlled trial of an intervention to reduce the rate of external ventricular drain infection. Neurosurgery. 2012. 71:
5. Konstantelias AA, Vardakas KZ, Polyzos KA, Tansarli GS, Falagas ME. Antimicrobial-impregnated and coated shunt catheters for prevention of infections in patients with hydrocephalus: A systematic review and meta-analysis. J Neurosurg. 2015. 122: 1096-112
6. Lackner P, Beer R, Broessner G, Helbok R, Galiano K, Pleifer C. Efficacy of silver nanoparticles-impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalus. Neurocrit Care. 2008. 8: 360-5
7. Lemcke J, Depner F, Meier U. The impact of silver nanoparticle-coated and antibiotic-impregnated external ventricular drainage catheters on the risk of infections: A clinical comparison of 95 patients. Acta Neurochir Suppl. 2012. 114: 347-50
8. Ramanan M, Lipman J, Shorr A, Shankar A. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect Dis. 2015. 15: 3-
9. Roethlisberger M, Moffa G, Fisch U, Wiggli B, Schoen S, Kelly C. Effectiveness of a chlorhexidine dressing on silver-coated external ventricular drain-associated colonization and infection: A prospective single-blinded randomized controlled clinical trial. Clin Infect Dis. 2018. 67: 1868-77
10. Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Michael Scheld W. 2017 infectious diseases society of America's clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017. 64: e34-65