- Department of Neurosurgery, Faculty of Medicine, Cairo University, Cairo, Egypt.
DOI:10.25259/SNI_118_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammad Elbaroody, Mohamed Adel Ghoneim, Ahmed El Fiki, Hesham Hozayen, Wael El-Mahdy. Short-term outcomes of endoscopic evacuation of supratentorial spontaneous intracerebral hematoma: Early experience from developing country. 28-Jun-2021;12:309
How to cite this URL: Mohammad Elbaroody, Mohamed Adel Ghoneim, Ahmed El Fiki, Hesham Hozayen, Wael El-Mahdy. Short-term outcomes of endoscopic evacuation of supratentorial spontaneous intracerebral hematoma: Early experience from developing country. 28-Jun-2021;12:309. Available from: https://surgicalneurologyint.com/surgicalint-articles/10927/
Abstract
Background: Minimally invasive endoscopic hematoma evacuation is a promising treatment option for intracerebral hemorrhage (ICH). However, the technique still needs improvement.
Methods: We report our early clinical experience of using this technique to evacuate supratentorial spontaneous intracerebral hematomas and evaluate its short-term outcomes.
Results: The study included 15 patients, basal ganglia hematoma was the most common location 62.5%, mean preoperative hematoma volume was 61.07 cc, mean ICH score was 3, and mean rate of hematoma evacuation was 89.27%. Factors that could be related to mortality were Glasgow Coma Score (GCS) on admission (P = 0.001), ICH score (P = 0.004); postoperative GCS (P P = 0.006); intraventricular extension (P = 0.001), and rate of evacuation (P = 0.001).
Conclusion: Endoscopic technique is a safe surgical option for evacuation of spontaneous supratentorial ICH. This minimally invasive technique could be helpful to provide better short-term outcomes for selected patients. However, in our experience, this minimally invasive technique did not change the outcome for cases presented with poor GCS on admission 4/15. Our results warrant a future prospective, randomized, controlled efficacy trial.
Keywords: Endoscopic, Hematoma, Hemorrhage, Intracerebral, Outcomes, Spontaneous
INTRODUCTION
Spontaneous intracerebral hemorrhage (ICH) represents approximately 15% of all strokes.[
The benefits of surgical evacuation for spontaneous ICH are controversial.[
Surgical options for evacuation of ICH include craniotomy, decompressive craniectomy, and minimally invasive procedures including stereotactic aspiration and endoscopic evacuation.[
In this prospective study, we report our early experience of endoscopic evacuation of spontaneous intracerebral hematoma. We discussed the short-term outcomes of the technique using the following variables: hematoma evacuation rate, operation time, Glasgow Coma Score (GCS), blood loss, rebleeding, epilepsy, hospital stay, and Glasgow Outcome Score (GOS).
MATERIALS AND METHODS
Study design
We retrospectively collected the data of 15 patients who underwent endoscopic evacuation of spontaneous supratentorial intracerebral hematoma between November 2018 and March 2020 in our institute.
Inclusion criteria were adult patients 18–70 years with GCS (4–14) and one of the following: (1) basal ganglia ICH with a hematoma volume >30 cc; or (2) subcortical hemorrhage > 30 cc with a significant mass effect (midline shift >5 mm and sulcus effacement); or (3) thalamic ICH with a hematoma volume >20 cc and acute hydrocephalus; or (4) combined type ICH or large hematoma occupying both the basal ganglia and the thalamus.
Exclusion criteria were as follows: age < 18 and > 70 years, ICH caused by intracranial tumors, aneurysms, trauma, infarction, or other intracranial lesions; basal ganglia ICH or subcortical hemorrhage <30 cc, thalamic hematoma <20 cc without hydrocephalus; multiple intracranial hemorrhages, infratentorial spontaneous ICH; coagulation disorders or history of anticoagulant drugs, infectious meningitis, and pulmonary or general infection; GCS < 4 or presence of clinical and radiologic signs of cerebral hernia or death, or severe systemic diseases such as heart, liver, lung, or kidney dysfunction.
In terms of short-term results, the hematoma volume was a critical pillar, patients with basal ganglia hematoma >30 cc and thalamic hematoma >20 cc could benefit from the endoscopic technique in the reported endoscopic series,[
Data collection
We collected demographic data such as gender and age, preoperative hematoma volume, intraventricular extension, ICH score, hematoma evacuation rate; operation time, GCS; blood loss, rebleeding; epilepsy, hospital stay, and GOS.
All patients underwent computed tomography (CT) brain at admission to localize the hematoma. Routine preoperative laboratories were done for all patients preoperatively.
We adopted the Tada Formula to calculate the hematoma volume, which is defined as V = a × b × c × 1/2, where “V” represents hematoma volume; “a” indicates the diameter of the largest area of the hematoma layer; “b” shows the upright diameter of the diameter of the largest area of the hematoma layer, and “c” is the layer thickness.[
Surgical technique
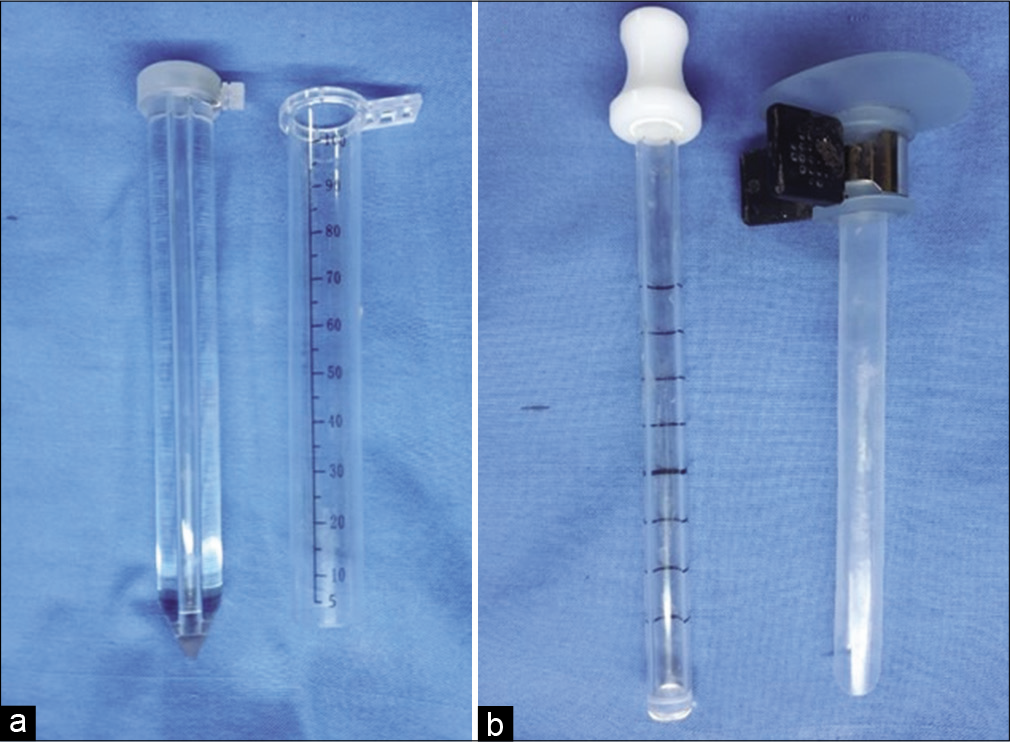
We made a linear/curved skin incision, an average of 5 cm in length in a fashion that could be extended to do open microsurgical evacuation if failed the endoscopic technique. The burr holes were designed according to the location of hematoma: for hematoma in basal ganglia, we used entry point 2–3 cm anterior to the coronal suture and 3–4 cm lateral to the midline, that is, lateral to Kocher’s point; for thalamic hematoma, we used entry posterior parietal entry point, that is, similar to Keen’s point but with different trajectory; for lobar hematoma, we used the shortest approach to the hematoma in relation to the surface of the skull. The diameter of the burr hole was 2.5–3 cm, in some cases, we were forced to widen it after we faced cortical vein after dural opening.
All patients were operated under general anesthesia, we used endoscopes 0 rod lens of 4 mm diameter 18 cm in length (Karl Storz, Tuttlingen, Germany).
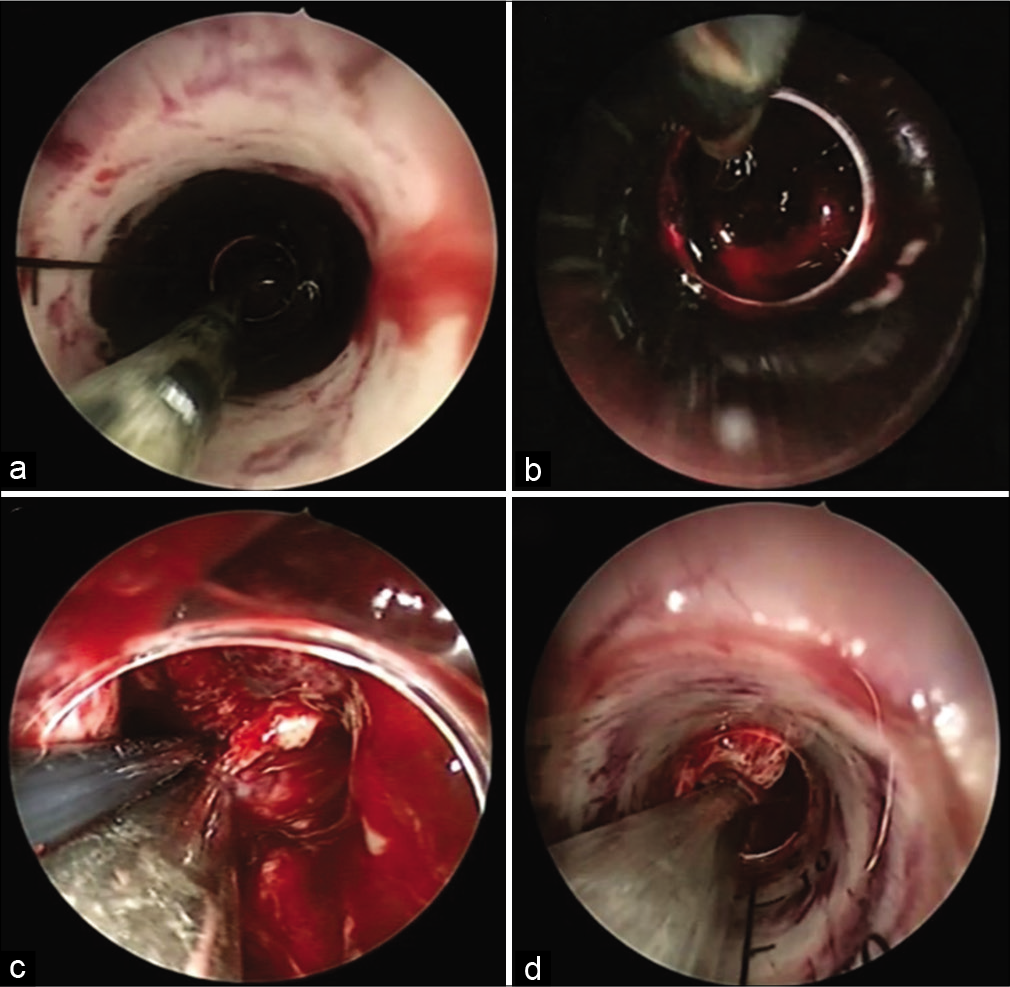
A 7 or 10 mm diameter suction tube with a coagulation apparatus was used to evacuate the hematoma and coagulate the vessel, and endoscopic bipolar was used alternatively in some cases. We used two endoscopic transparent sheathes interchangeably, a transparent plastic sheath (TianXinFu [Beijing] Medical Appliance Co., Ltd., China) and NeuroPort (Olympus Corp., Tokyo) [
We did not try to remove the clot adherent to the cavity wall, and we sometimes used saline irrigation as a trial to liquefy the hematoma to make it easy to be aspirated. Most bleeding from these perforators stopped after gentle compression with cotton and irrigation for 5 min. When a bleeder was identified with an endoscope, coagulation was preferred using a suction tube with a coagulation apparatus [
After adequate evacuation of hematoma, we closed the wound after the blood pressure was elevated more than 150/90 and maintained for 5 min. We stitched the dura then the periosteal layer followed by closure of the skin in layers. We did not place subgaleal drain in any case, and we used placed External Ventricular Drain (EVD) in two cases of ICH with intraventricular extension.
Postoperative care and assessment
All patients were transferred to the intensive care unit (ICU) where blood pressure was maintained on the high normal range, and antiepileptic drugs and antibiotics were continued. Routine CT brain was performed on the same postoperative day for all patients because all patients are transferred to ICU without a trial of extubation to avoid an unpredictable rise in the blood pressure in the immediate postoperative recovery.
We calculated the hematoma evacuation as a percentage: (postoperative hematoma volume/preoperative hematoma volume). The length of hospital stay was documented for each patient. Assessment of clinical outcome at discharge from the hospital was done using GOS.
[
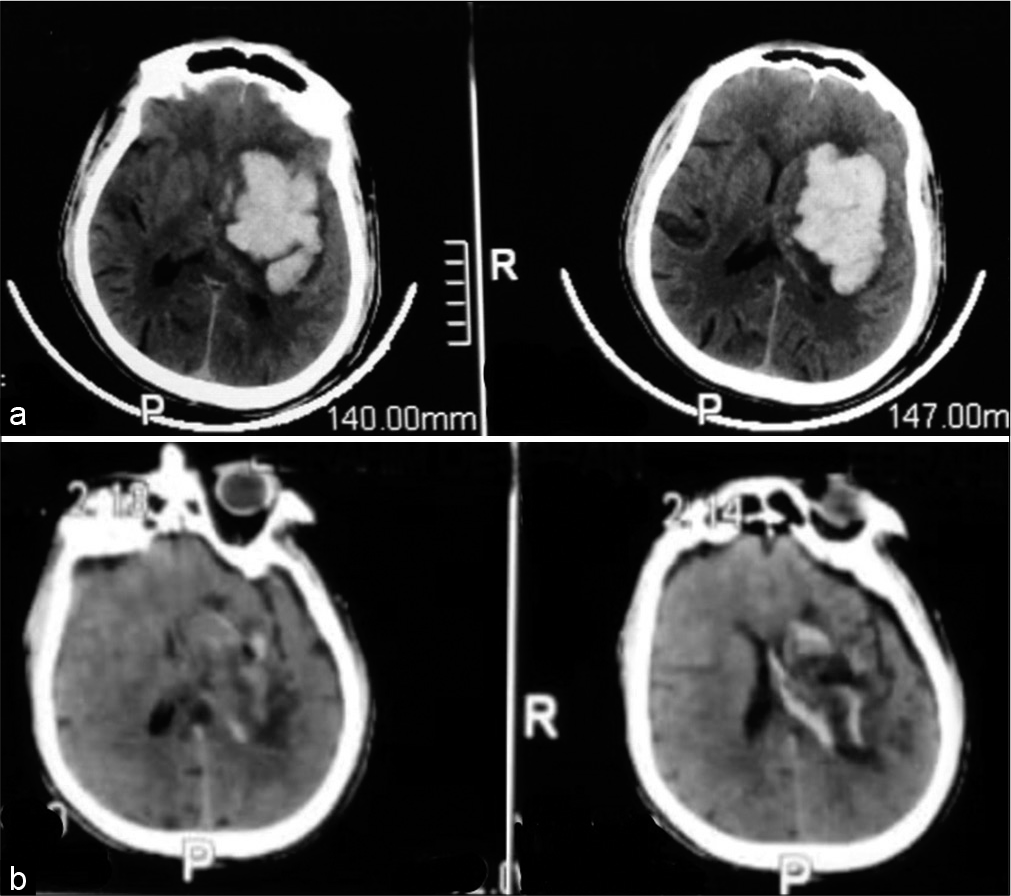
Figure 3:
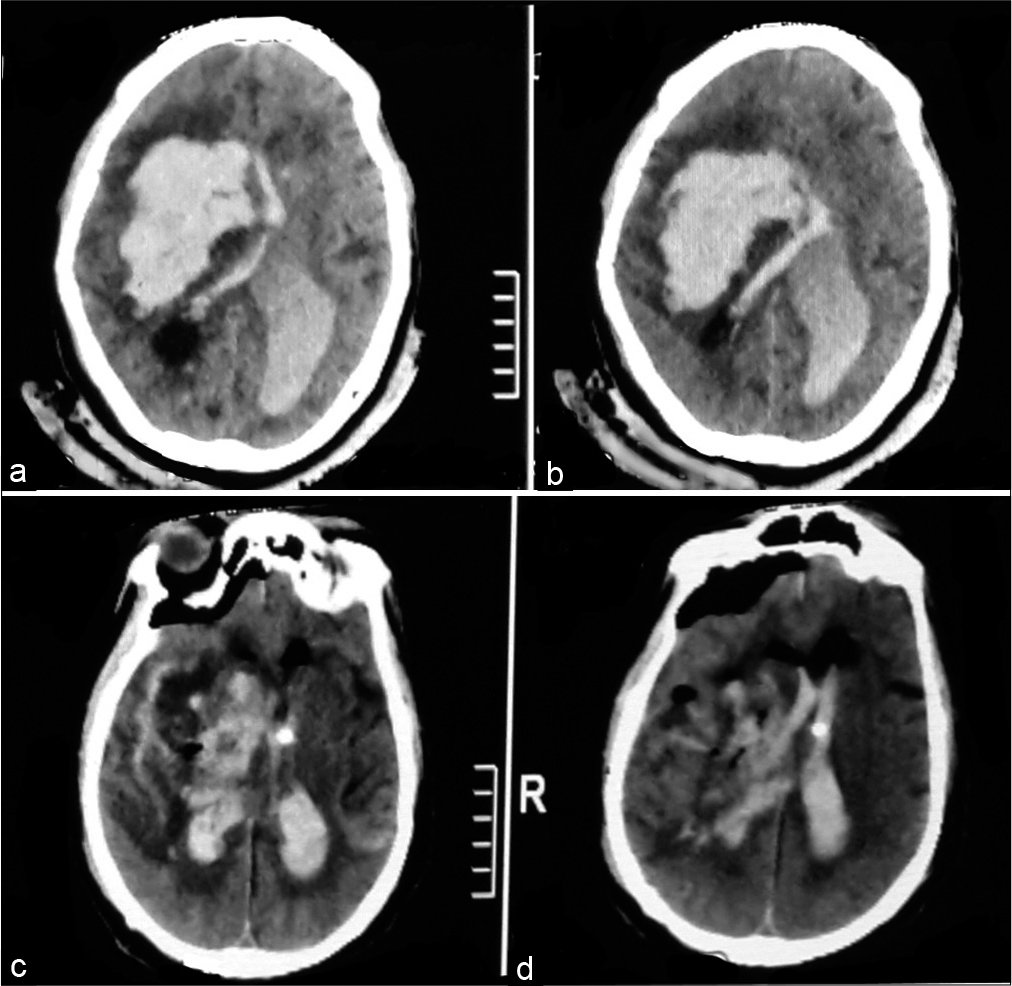
A 63-year-old male patient presented with Glasgow Coma Score 8, computed tomography (CT) brain on admission (a) showed left basal ganglia intracerebral hemorrhage, 42 cc. Postoperative CT brain (b) revealed complete hematoma evacuation. The patient was discharged from the hospital after 8 days with Glasgow Outcome Score 4.
Figure 4:
A 62-year-old female patient presented with Glasgow Coma Score 12, computed tomography (CT) brain on admission (a) showed left thalamic intracerebral hemorrhage, 30 cc. Postoperative CT brain (b) revealed complete hematoma evacuation. The patient had a stable conscious level for 15 days, unfortunately, she was infected with COVID-19 +ve and died from a chest infection.
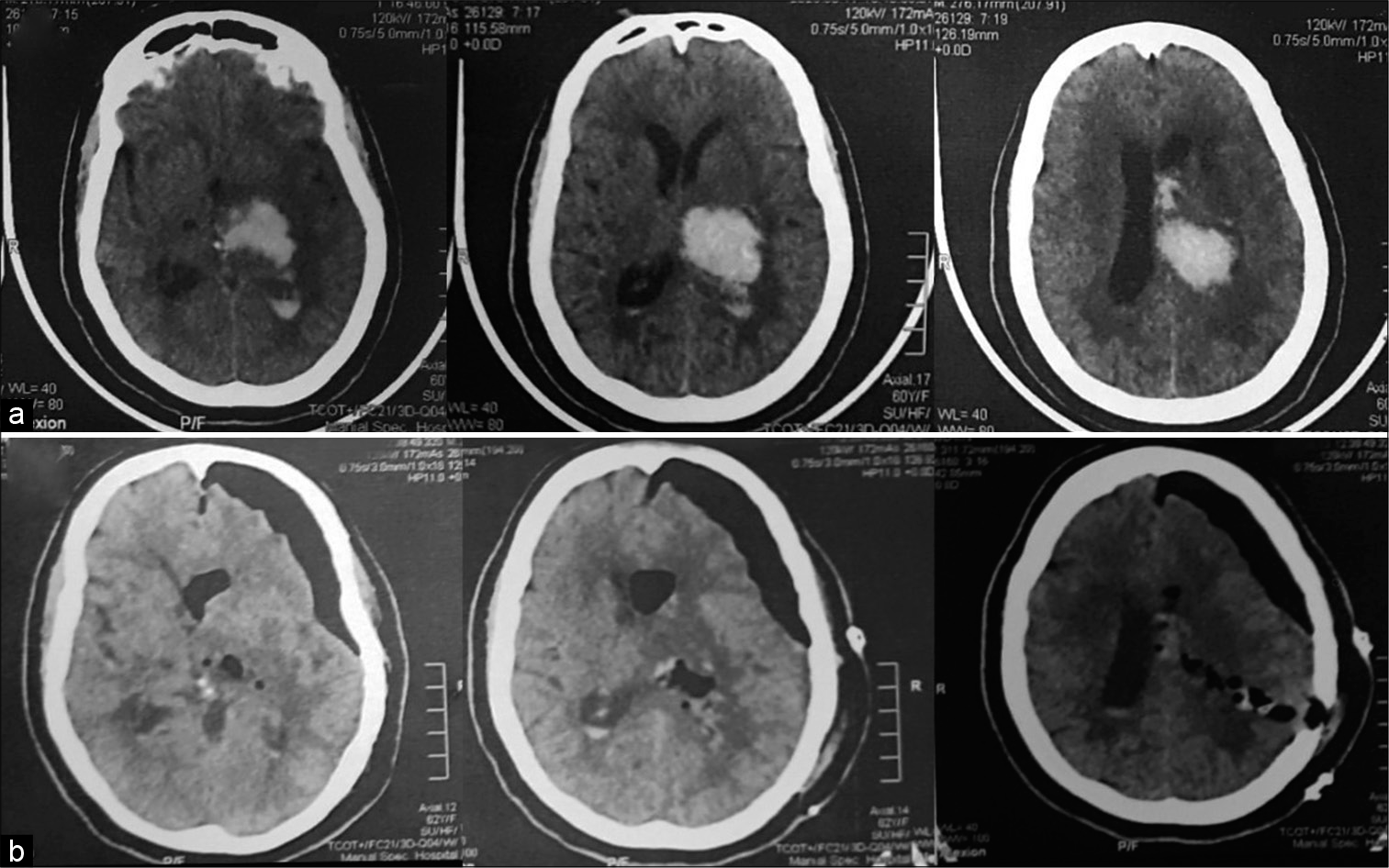
Figure 5:
A 56-year-old male patient presented with Glasgow Coma Score 7, computed tomography (CT) brain on admission (a) showed left parietal intracerebral hemorrhage, 66 cc. Postoperative CT brain (b) revealed complete hematoma evacuation. The patient was discharged from the hospital after 6 days with Glasgow Outcome Score 4.
Figure 6:
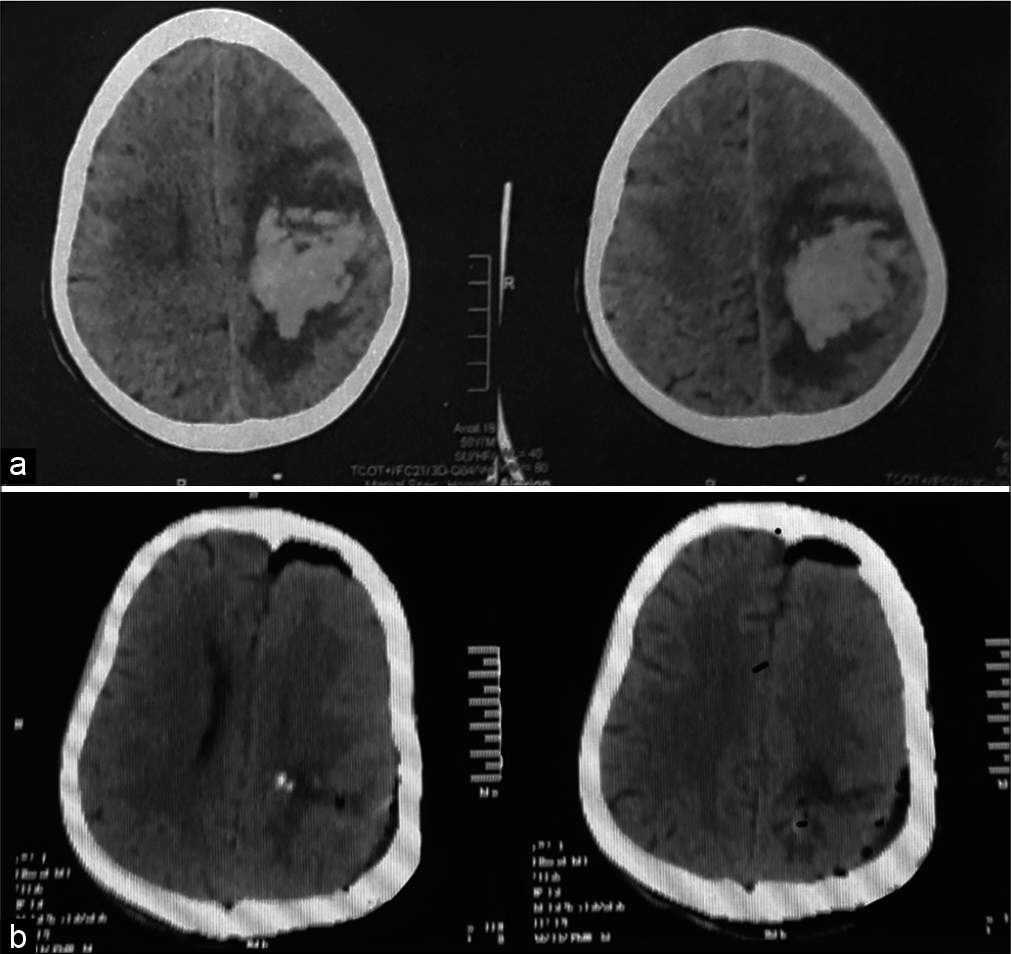
A 59-year-old male patient presented with Glasgow Coma Score 4, computed tomography (CT) brain on admission (a) showed right basal ganglia and thalamic intracerebral hemorrhage, 80 cc. Postoperative CT brain (b) revealed good hematoma evacuation with left frontal external ventricular drain. The patient stayed in the hospital for 3 days then died.
Statistical methods
Data were coded and entered using the Statistical Package for the Social Sciences version 26 (IBM Corp., Armonk, NY, USA). Data were summarized using mean, standard deviation, median, minimum and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were done using the nonparametric Mann–Whitney U-test. For comparison of serial measurements (before and after) within each patient, the nonparametric Wilcoxon signed-rank test was used.[
RESULTS
General preoperative data
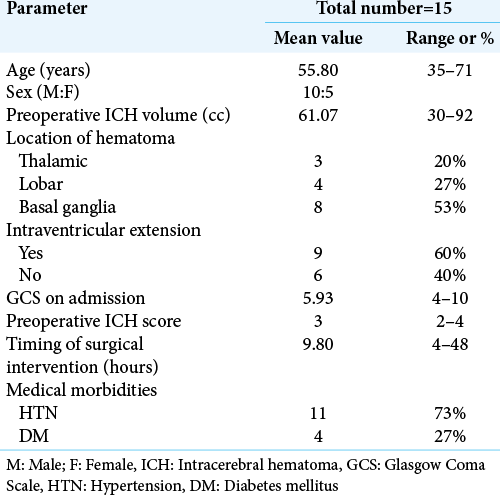
The mean age for the 15 patients who underwent the evacuation was 55.8 ± 10.09 years. Ten were male and five were female. Eleven patients 73.3% were known to be hypertensive at the time of presentation, while 26.7% of the patients were not. DM was found as a comorbidity in 26.7% of patients.
The mean GCS on admission was 5.93, three patients admitted with GCS 13, 12, and 13 for conservative treatment, then they deteriorated in conscious level down to 5, 5, and 9, respectively, for whom endoscopic evacuation was done. The most common location was in basal ganglia in eight patients 53.3%. The mean hematomas size on preoperative CT was 61.07 ± 18.52 cc. The incidence of IVH on the initial CT scan was 60%, and we placed EVD in two patients. The mean ICH score for all patients included was 3 ± 1. The mean time interval between ictus and surgery was 9.8 h ± 11.05 h. The shortest time interval to surgery was 4 h and the longest was 48 h. The mean duration of surgery was 55 min ± 3.18 min. The longest surgery was 73 min. [
Short-term outcomes
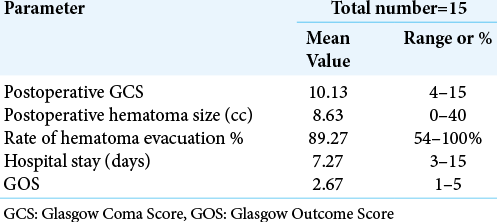
The mean postoperative GCS was 10.13 (P = 0.007). Two patients were operated with GCS 5 for both of them and postoperative showed improvement on GCS to 13, they stayed for 15 and 12 days in ICU, respectively, however; one patient died from COVID-19 and the other died from sepsis. The mean postoperative hematoma volume was 8.63 ± 11.11 cc (P = 0.001). The mean evacuation rate was 89.27% of its preoperative volume, one patient with an evacuation rate of 54%, reoperated with microscopic technique. The mean duration of postoperative hospital stay was 7.27 days. The mean postoperative GOS was 2.67. [
Postoperative complications
Mortality
The one patient occurred in a single case, the hematoma was evacuated with 54% and no immediate improvement in conscious level, the patient operated on by microsurgical evacuation.
DISCUSSION
The surgical treatment options for ICH consist mainly of three methods; conventional craniotomy, endoscopic evacuation, and stereotactic evacuation.
In 1989, Auer et al. conducted the first randomized controlled trial about conservative treatment versus endoscopic surgery for spontaneous ICH.[
Timing of surgery
There is no clear evidence at present time indicates that ultra-early removal of supratentorial ICH improves functional outcomes or mortality rates.[
Ma et al. reported in their endoscopic series a mean time between ictus and surgery were 4–50 h with no statistical significance (P = 0.28),[
Nishihara et al. recommended performance of the endoscopic technique within 24 h after onset because intracerebral hematoma usually starts to harden about 24 h after onset, and 48 h later, it cannot be evacuated with a suction tube.[
We noticed also that the shorter time to ictus the easier it to be sucked, and as time passes the hematoma becomes more organized and we used irrigation with saline as a trial to dissolve it. Concerning mortality, there was no statistical significance between the timing of surgery and mortality (P = 0.536).
Preoperative GCS
Many published series of the endoscopic technique included patients with GCS ≥ 4.[
Hematoma volume and evacuation rate
The ICH volume is strongly associated with the outcome of the ICH.[
The evacuation rate reported by the published endoscopic series ranged from 80% to 99%.[
Intraventricular extension
Intraventricular hemorrhage was associated with impaired consciousness on admission[
ICH score
Hemphill et al. published the ICH score as a grading scale for ICH in 2001, they noticed that all six patients with an ICH score of 5 died.[
Surgical technique
Craniotomy has been used traditionally as the first option for large spontaneous ICH, sometimes with decompressive flap and duroplasty. The craniotomy provides a good view, immediate brain decompression, and adequate hemostasis, however, it is associated with more blood loss, increase in operation time, and risk of infection.[
Li et al. found that stereotactic aspiration and endoscopic surgery were superior in both short-term and long-term outcomes than craniotomy for the treatment of spontaneous supratentorial lobar ICH.[
Sun et al. found no difference between keyhole endoscopy and craniotomy regarding mortality and rebleeding rate.[
In our series, the unfavorable outcome in the form of GOS 1 (53.3%) is comparable to patients who underwent microsurgical evacuation using craniotomy in another center in Egypt with GOS 1–2 (56.1%).[
Complications
Endoscope-assisted ICH evacuation performed in the early stage was associated with a minimal rebleeding rate (0– 3.3%) compared with the traditional craniotomy method (5–10%).[
GOS
In the published endoscopic series, Li et al. reported a mean GOS 3.4 ± 1.5 in 22 patients,[
In our series, the mean postoperative GOS was 2.67 (1–5), four patients discharged without deficit 26.7% (GOS 5), three patients discharged with weakness 20% (GOS 4), and eight patients died 53.3% (GOS 1).
Mortality
There are a lot of factors reported in the literature related to poor outcome or mortality in spontaneous ICH: low GCS score on admission, large hematoma size, intraventricular extension of blood, and patient’s age, gender, deep coma, ataxic respiration, abnormal pupils, and acute hypertension.[
GCS was an important predictor of the level of disability and mortality in many published series.[
Factors that could be linked to mortality: GCS on admission (P = 0.001), ICH score (P = 0.004), postoperative GCS (P < 0.001), postoperative hematoma volume (P = 0.006), intraventricular extension (P = 0.001), and percentage of hematoma evacuation (P = 0.001).
We obtained comparable results in timing of intervention, length of surgery, postoperative hematoma volume, and hematoma evacuation in our early experience with the endoscopic technique. However, our series’ mortality is much higher than that reported in the literature, and this is mainly due to low GCS at presentation 4/15. Five patients accounted for 62.5% of the total mortality.
In certain patients, the endoscopic technique can provide optimal results that are comparable to the open microsurgical technique. Our findings call for a potential prospective, randomized, controlled efficacy trial that compares GCS outcomes in different groups.
Study limitations
We agree that the preliminary findings of this retrospective study are limited. The sample size was insufficient to provide reliable evidence for clinical use. Many patients with a GCS score of 4 and a mean time to surgery of 9.5 h from ictus have a poor prognosis, which may explain why our series had a higher mortality rate than other series, due to poor GCS at admission 4/15 due to late arrival at the hospital and hence a higher ICH score with corresponding higher mortality.
CONCLUSION
Endoscopic technique is a safe surgical option for evacuation of spontaneous supratentorial ICH. For certain patients, this minimally invasive approach can be beneficial in terms of improving short-term outcomes. In our experience, this minimally invasive procedure had little impact on the outcome of cases with a low GCS at the time of admission, that is, 4/15. This technique necessitates a steep learning curve, implying the acquisition of skills. As a result, familiarity with the endoscope and associated instrumentation is the most effective way to achieve a better result with less complication. Our findings call for a prospective, randomized, controlled efficacy trial in the future.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
For this retrospective study, formal consent is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aghi MK, Ogilvy CS, Carter BS.editors. Surgical management of intracerebral hemorrhage. Schmidek and Sweet Operative Neurosurgical Techniques. Amsterdam: Elsevier; 2012. p. 823-36
2. An SJ, Kim TJ, Yoon B-W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017. 19: 3-10
3. Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: A randomized study. J Neurosurg. 1989. 70: 530-5
4. Beynon C, Schiebel P, Bösel J, Unterberg AW, Orakcioglu B. Minimally invasive endoscopic surgery for treatment of spontaneous intracerebral haematomas. Neurosurg Rev. 2015. 38: 421-8
5. Bhaskar MK, Kumar R, Ojha B, Singh SK, Verma N, Verma R. A randomized controlled study of operative versus nonoperative treatment for large spontaneous supratentorial intracerebral hemorrhage. Neurol India. 2017. 65: 752
6. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-touse predictor of 30-day mortality. Stroke. 1993. 24: 987-93
7. Chan YH. Biostatistics 102: Quantitative data-parametric and non-parametric tests. Singapore Med J. 2003. 44: 391-6
8. Chan YH. Biostatistics 103: Qualitative data-tests of independence. Singapore Med J. 2003. 44: 498-503
9. Cho DY, Chen CC, Chang CS, Lee WY, Tso M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: Comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol. 2006. 65: 547-55
10. Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006. 66: 1175-81
11. Gregson BA, Broderick JP, Auer LM, Batjer H, Chen XC, Juvela S. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012. 43: 1496-504
12. Gregson BA, Mendelow AD, STICH Investigators. David. International variations in surgical practice for spontaneous intracerebral hemorrhage. Stroke. 2003. 34: 2593-7
13. Hallevi H, Albright KC, Aronowski J, Barreto AD, MartinSchild S, Khaja AM. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology. 2008. 70: 848-52
14. Helweg-Larsen S, Sommer W, Strange P, Lester J, Boysen G. Prognosis for patients treated conservatively for spontaneous intracerebral hematomas. Stroke. 1984. 15: 1045-8
15. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score. Stroke. 2001. 32: 891-7
16. Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American heart association/ American stroke association. Stroke. 2015. 46: 2032-60
17. Intiso D, Stampatore P, Zarrelli MM, Guerra GL, Arpaia G, Simone P. Incidence of first-ever ischemic and hemorrhagic stroke in a well-defined community of southern Italy, 1993-1995. Eur J Neurol. 2003. 10: 559-65
18. khallaf M, Abdelrahman M. Surgical management for large hypertensive basal ganglionic hemorrhage: Single center experience. Egypt J Neurosurg. 2019. 34: 19
19. Kumral E, Kocaer T, Ertübey NO, Kumral K. Thalamic hemorrhage. A prospective study of 100 patients. Stroke. 1995. 26: 964-70
20. Kuo LT, Chen CM, Li CH, Tsai JC, Chiu HC, Liu LC. Early endoscope-assisted hematoma evacuation in patients with supratentorial intracerebral hemorrhage: Case selection, surgical technique, and long-term results. Neurosurg Focus. 2011. 30: E9
21. Li Y, Yang R, Li Z, Yang Y, Tian B, Zhang X. surgical evacuation of spontaneous supratentorial lobar intracerebral hemorrhage: Comparison of safety and efficacy of stereotactic aspiration, endoscopic surgery, and craniotomy. World Neurosurg. 2017. 105: 332-40
22. Lisk DR, Pasteur W, Rhoades H, Putnam RD, Grotta JC. Early presentation of hemispheric intracerebral hemorrhage: Prediction of outcome and guidelines for treatment allocation. Neurology. 1994. 44: 133
23. Ma L, Hou Y, Zhu R, Chen X. Endoscopic evacuation of basal ganglia hematoma: Surgical technique, outcome, and learning curve. World Neurosurg. 2017. 101: 57-68
24. Miller CM, Vespa P, Saver JL, Kidwell CS, Carmichael ST, Alger J. Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol. 2008. 69: 441-6
25. Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T. Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: Preliminary study in comparison with craniotomy. J Stroke Cerebrovasc Dis. 2011. 20: 208-13
26. Nishihara T, Morita A, Teraoka A, Kirino T. Endoscopy-guided removal of spontaneous intracerebral hemorrhage: Comparison with computer tomography-guided stereotactic evacuation. Childs Nerv Syst. 2007. 23: 677-83
27. Nishihara T, Nagata K, Tanaka S, Suzuki Y, Izumi M, Mochizuki Y. Newly developed endoscopic instruments for the removal of intracerebral hematoma. Neurocrit Care. 2005. 2: 67-74
28. Nishihara T, Teraoka A, Morita A, Ueki K, Takai K, Kirino T. A transparent sheath for endoscopic surgery and its application in surgical evacuation of spontaneous intracerebral hematomas: Technical note. J Neurosurg. 2000. 92: 1053-5
29. Qureshi AI, Safdar K, Weil J, Barch C, Bliwise DL, Colohan AR. Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage. Stroke. 1995. 26: 1764-7
30. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001. 344: 1450-60
31. Sun G, Li X, Chen X, Zhang Y, Xu Z. Comparison of keyhole endoscopy and craniotomy for the treatment of patients with hypertensive cerebral hemorrhage. Medicine (Baltimore). 2019. 98: e14123
32. Tada A, Hisada K, Suzuki T, Kadoya S. Measurement volume of intracranial hematoma by computed tomography (author’s transl). No Shinkei Geka. 1981. 9: 251-6
33. Wang WH, Hung YC, Hsu SP, Lin CF, Chen HH, Shih YH. Endoscopic hematoma evacuation in patients with spontaneous supratentorial intracerebral hemorrhage. J Chin Med Assoc. 2015. 78: 101-7
34. Yokosuka K, Uno M, Hirano K, Toi H, Matsuzaki K, Matsubara S. Freehand technique for putaminal hemorrhage. Neurol Med Chir. 2011. 51: 543-6
35. Zhao XH, Zhang SZ, Feng J, Li ZZ, Ma ZL. Efficacy of neuroendoscopic surgery versus craniotomy for supratentorial hypertensive intracerebral hemorrhage: A meta-analysis of randomized controlled trials. Brain Behav. 2019. 9: e01471
36. Zhao Y, Chen X. Endoscopic treatment of hypertensive intracerebral hemorrhage: A technical review. Chronic Dis Transl Med. 2016. 2: 140-6