- Department of Neurosurgery, Juntendo University Shizuoka Hospital, Izunokuni, Shizuoka, Japan.
DOI:10.25259/SNI_872_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Naohide Fujita, Hideaki Ueno, Mitsuya Watanabe, Yasuaki Nakao, Takuji Yamamoto. Significance of endoscopic hematoma evacuation in elderly patients with spontaneous putaminal hemorrhage. 30-Mar-2021;12:121
How to cite this URL: Naohide Fujita, Hideaki Ueno, Mitsuya Watanabe, Yasuaki Nakao, Takuji Yamamoto. Significance of endoscopic hematoma evacuation in elderly patients with spontaneous putaminal hemorrhage. 30-Mar-2021;12:121. Available from: https://surgicalneurologyint.com/surgicalint-articles/10690/
Abstract
Background: The efficacy of endoscopic surgery for spontaneous intracerebral hemorrhages (ICH) has been previously reported, but differences in the effect between early and late elderlies remain unclear.
Methods: Ninety-seven patients diagnosed with putaminal hemorrhage (age, ≥65 years; hematoma volume, ≥30 mL) were included in this retrospective analysis and separated into three treatment groups: craniotomy surgery (CR), endoscopic surgery (EN), and non-surgical (NS) groups. The patients were additionally subdivided into two groups according to age: patients aged 65–74 years (“early elderlies”) and patients aged ≥75 years (“late elderlies”). Their clinical data and outcomes at discharge were compared using statistical analyses.
Results: The CR and the EN groups were associated with lower mortality rates (P P = 0.007), and lower National Institutes of Health Stroke Scale (NIHSS; P = 0.029) compared to the NS group. Early elderlies in the CR and EN groups with ICH scores of 3 also had significantly better outcomes (P = 0.001). The proportion of patients with mRS ≤ 4 was highest in the early elderlies of the EN group (P = 0.553). Although significant differences in the change of NIHSS scores between the early and late elderlies was not observed, significantly improved NIHSS scores were observed in the EN group compared to the NS group, even in the late elderlies (P = 0.037).
Conclusion: The evacuation of deep-seated intracranial hematomas using the endoscope might improve functional outcomes and mortality, regardless of age.
Keywords: Aging society, Endoscope, Minimally invasive surgery, Putaminal hemorrhage, Spontaneous intracerebral hemorrhage
INTRODUCTION
The aging of society has progressed to become one of the most prominent challenges in Japan. For elderly patients, it is important to consider the most suitable treatment. Minimally invasive surgery (MIS) has attracted attention as a treatment option for various conditions due to its broad application in elderly patients. Spontaneous intracerebral hemorrhage (ICH) accounts for approximately 15% of all strokes and is associated with high rates of morbidity and mortality.[
The aim of this study was to analyze our institutional cases of putaminal hemorrhage and evaluate the effectiveness of endoscopic surgery, with a focus on the differences between early and late elderlies.
MATERIALS AND METHODS
Patient characteristics
This study was approved by the Ethics Committee of Institutional Review Board (Ethics No-675, the requirement for informed consent was waived). We reviewed our institutional cases of putaminal hemorrhage that was diagnosed using computed tomography (CT) imaging and had been treated at our hospital between 2005 and 2016. Eligible patients were aged 65 years or older; we defined those under 75 years of age as “early elderlies,” and the remainder as “late elderlies.” Hematoma volume was calculated using CT imaging with the formula ABC/2,[
A total of 97 cases of putaminal hemorrhage were enrolled in this study. These cases were differentiated into three groups: a conventional surgery group with craniotomy surgery group (CR group, n = 34), an endoscopic surgery (EN group, n = 38), and a non-surgical group (NS group, n = 25).
Endoscopic surgery
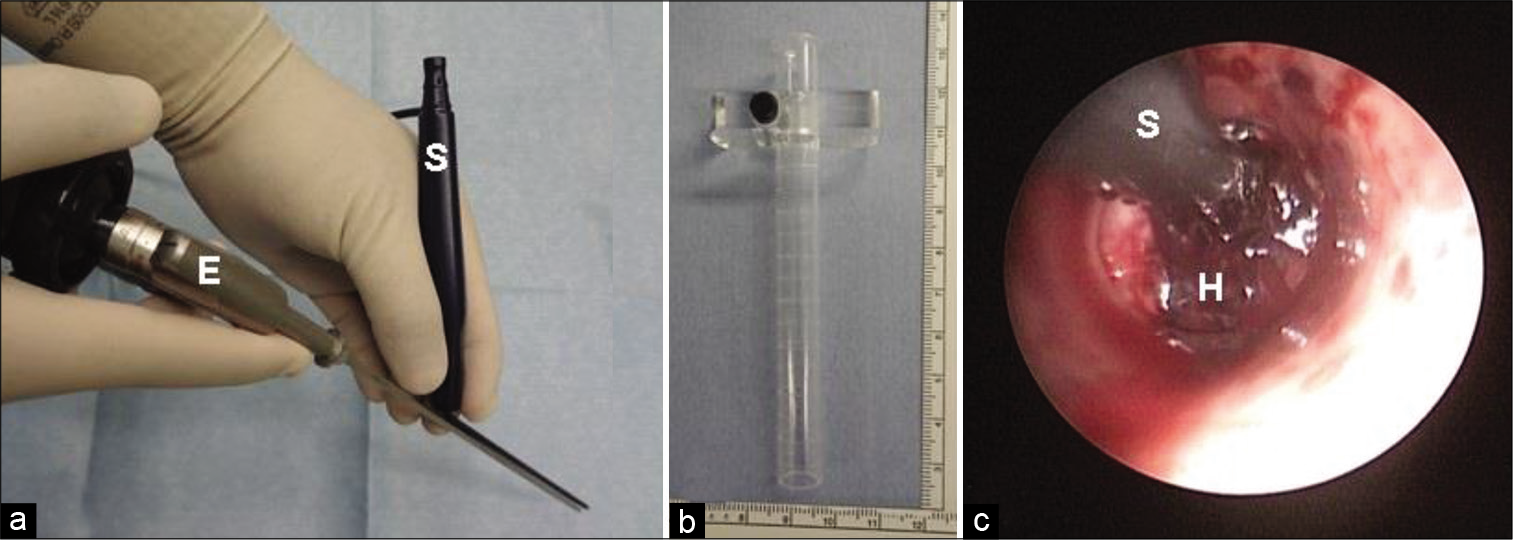
The endoscopic surgery was performed by two expert neuroendoscopists who were certified by the Japanese Society of Neuroendoscopy. Three surgical instruments were used for this endoscopic procedure [
The patient’s head was positioned in a neutral position under general anesthesia. A single burr hole was usually made at Kocher’s point for entry of the endoscope. The clear guide sheath was carefully inserted into the hematoma cavity. Both the endoscope and the suction tube were inserted into the sheath, and the hematoma clot was evacuated under endoscopic guidance by manipulating them [
Hematoma removal with conventional craniotomy
In our craniotomy surgery approach, the putaminal hemorrhage was removed through a conventional frontotemporal approach. The transsylvian fissure approach or trans-middle temporal gyrus approach was used to remove the hematoma. In all cases, the microscope was used during intracerebral procedures.
Conservative treatments
All cases enrolled in this study received the best medical treatment, which included blood pressure control under 140 mmHg with nicardipine hydrochloride, and tranexamic acid was used as a hemostatic agent immediately following putaminal hemorrhage diagnosis using CT scan. In some cases, glycerol was used in the presence of widespread cerebral edema. Although airway management and respiratory support were used if required, all cases were subsequently independent of respiratory support except mortal cases. In addition, all cases received anti-thrombotic management using calf pumps, rehabilitation therapy, and nutritional management as early as possible, depending on their neurological deficit. If patients exhibited symptomatic delayed hydrocephalus, a ventriculoperitoneal (VP) shunt surgery was performed.
Statistical analysis
All statistical analyses in this study were performed using SPSS version 22 (SPSS IBM Corp., Armonk, NY, USA). Statistical comparisons were performed using one-way analysis of variance (ANOVA), Pearson’s Chi-square test, Mann–Whitney U test, and the Kruskal–Wallis test, where appropriate. In this study, statistical significance was confirmed when the P < 0.05. Modified Rankin Scale (mRS) and National Institutes of Health Stroke Scale (NIHSS) scores were used to compare outcomes in patients. The changes in NIHSS score between admission and discharge were calculated by subtracting the NIHSS score at discharge from that at admission. Each analysis was performed separately for each age group to evaluate the difference between the early and late elderly groups. In addition, we compared outcomes for each ICH score group.
RESULTS
Patient background
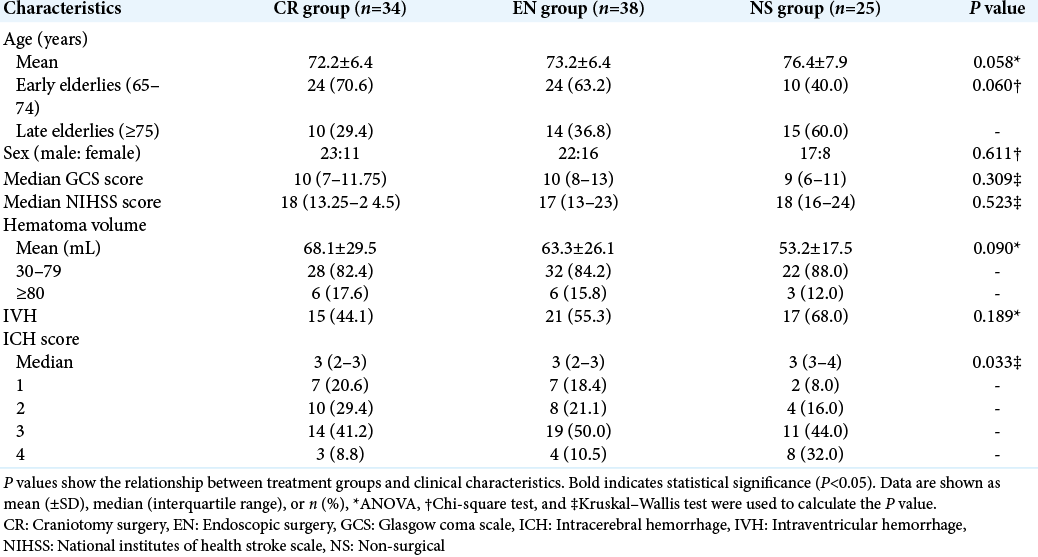
The baseline patient characteristics are shown in [
Outcome
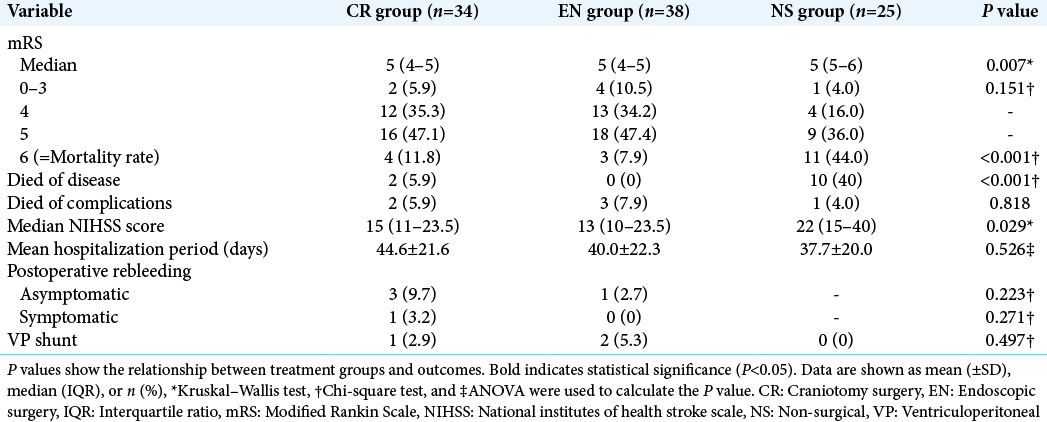
The results of the comparison of outcomes are shown in [
[
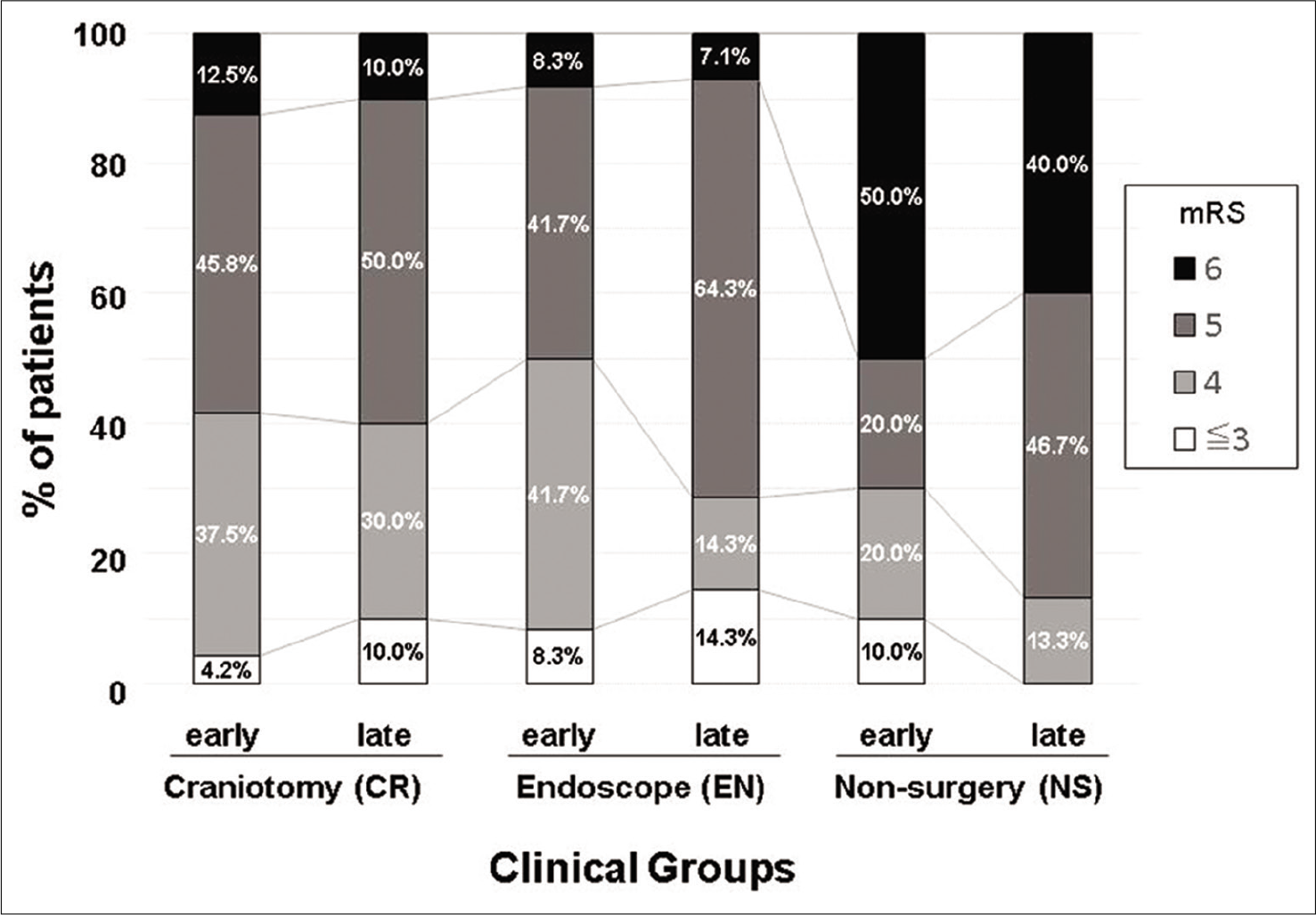
Figure 2:
Clinical outcome by mRS. Distribution of mRS at discharge. Bar graphs are shown by treatment and age groups. The CR and EN groups had lower mRS scores than the NS group, even after dividing patients into each age group. CR: Craniotomy surgery group, EN: Endoscopic surgery group, mRS: Modified Rankin Scale, NS: Non-surgical group.
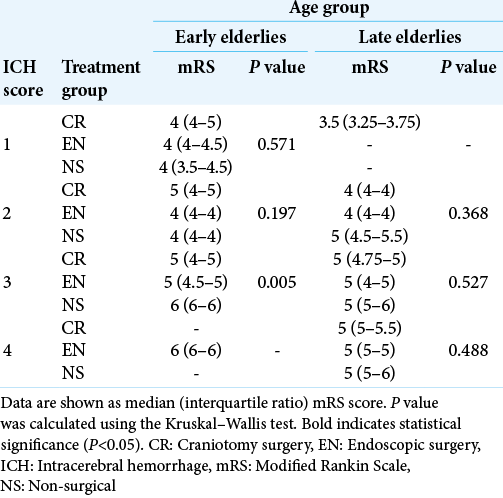
In addition, we performed statistical analysis according to each ICH score; the results are listed in [
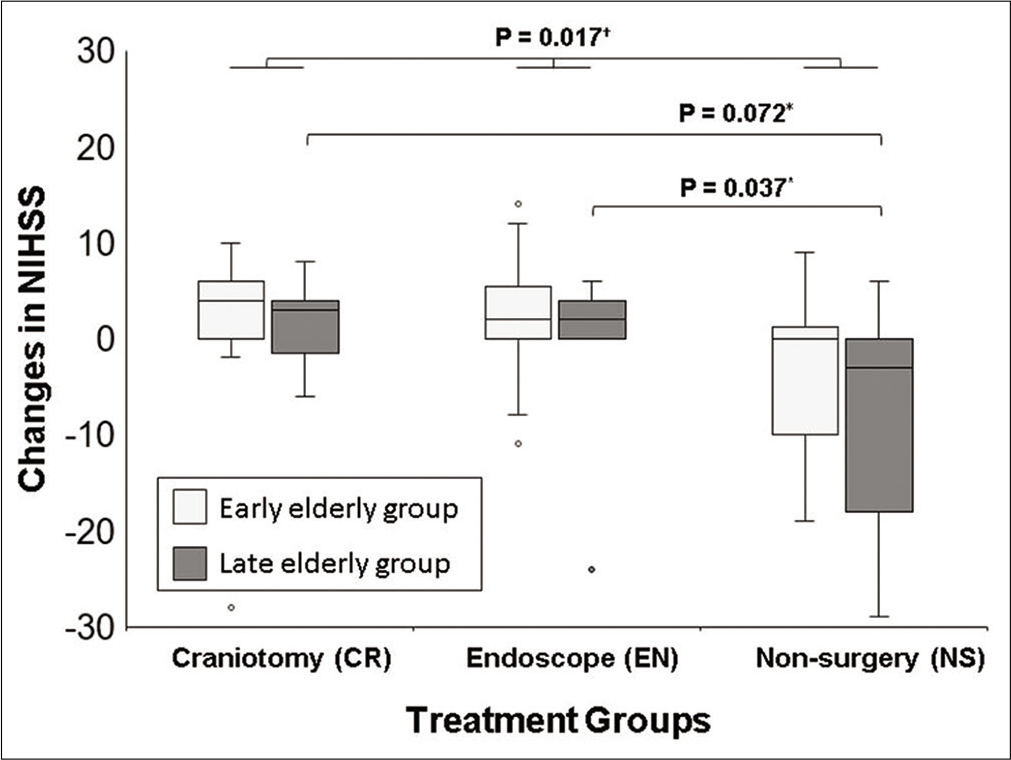
Finally, the comparisons of the changes in NIHSS scores between admission and discharge are shown in [
Figure 3:
Comparison of improvement in the NIHSS score following treatment. Box and whisker plot showing changes in NIHSS from admission to discharge for each treatment and age group. The center lines denote the median value, while the boxes contain the 25th–75th percentiles of data. The whiskers mark the largest value within 1.5 times the IQR above the 75th percentile, or the smallest value within 1.5 times the IQR below the 25th percentile. Outliers are marked as dots. The changes in NIHSS were statistically different in each group (P=0.017). In the late elderly group, the improvement of NIHSS in the EN group was significantly greater than that in the non-surgical group (P=0.037). *Mann–Whitney U test, †Kruskal–Wallis test. CR: Craniotomy surgery group, Early: Early elderly group, EN: Endoscopic surgery group, Late: Late elderly group, NIHSS: National institutes of health stroke scale, IQR: Interquartile range.
DISCUSSION
Surgical indication for ICH
At present, a benefit for surgery with conventional craniotomy for ICH has not been demonstrated; for example, the STICH I and II trials showed almost identical outcomes in surgically and medically managed cases.[
Advantages of endoscopic surgery in MIS
The MISTIE III trial, a representative randomized control trial using MIS,[
In addition, endoscopic surgery looks more advantageous with regard to hemostasis. More postoperative rebleeding was identified in 32% of MIS group patients in MISTIE III study, although it was limited to asymptomatic cases, while MIS did not increase symptomatic patients. Similar to previous findings, our current study showed that endoscopic surgery did not increase the frequency of postoperative bleeding compared to conventional open surgery.[
There are other advantages to endoscopic treatment, one of which is its potential application in hemorrhages in other locations. In the present study, the rate of IVH was the highest in the EN group; nevertheless, outcomes were better in the EN group, and this may be collateral evidence that endoscopic treatment is effective for IVH as well. Previous reports have shown the efficacy of endoscopic surgery for thalamic hemorrhage, cerebellar hemorrhage, and IVH.[
Application of endoscopic surgery for late elderlies
Age is an important factor in the prognosis of ICH patients.[
Limitations
One critical limitation of this study is that this study incurred a selective bias as this is a retrospective study, and the final decision on the surgical method was made by the neurosurgeon in charge of each patient. However, we suspect that the greatest cause of the selective bias is that family members of the more severe patients were more likely to refuse surgical intervention. The NS group showed higher median age, hematoma volume, frequency of IVH, and ICH score, but lower GCS score. In addition, the case numbers were relatively small. In total, 97 cases were enrolled in this study, yet more data are required to subdivide and analyze them accurately. Finally, long-term follow-up was not available in this study; thus, the improvement in the outcomes of patients following surgery may have been overlooked. Therefore, prospective studies are warranted to accumulate larger clinical datasets over a longer period of time, including follow-up.
CONCLUSION
We performed a retrospective analysis of 97 cases of putaminal hemorrhage. From this analysis, both conventional surgery with craniotomy and endoscopic surgery might contribute for better mortality rates as well as mRS and NIHSS scores, especially when patients are early elderlies with an ICH score of 3. Moreover, the late elderlies did not have different outcomes from early elderlies; therefore, late elderlies could deserve to undergo surgical intervention as well as early elderlies, and endoscopic surgery might be the best option for this group. Further research is warranted to accurately assess the effect of endoscopic surgery in this group.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Cai Q, Zhang H, Zhao D, Yang Z, Hu K, Wang L. Analysis of three surgical treatments for spontaneous supratentorial intracerebral hemorrhage. Medicine (Baltimore). 2017. 96: e8435
2. Coleman ER, Moudgal R, Lang K, Hyacinth H, Awosika OO, Kissela BM. Early rehabilitation after stroke: A narrative review. Curr Atheroscler Rep. 2017. 19: 59
3. Fiorella D, Arthur A, Bain M, Mocco J. Minimally invasive surgery for intracerebral and intraventricular hemorrhage: Rationale, review of existing data and emerging technologies. Stroke. 2016. 47: 1399-406
4. Fiorella D, Arthur AS, Mocco JD. 305 the INVEST trial: A randomized, controlled trial to investigate the safety and efficacy of image-guided minimally invasive endoscopic surgery with Apollo vs best medical management for supratentorial intracerebral hemorrhage. Neurosurgery. 2016. 63: 187
5. Fu CH, Wang N, Chen HY, Chen QX. Endoscopic surgery for thalamic hemorrhage breaking into ventricles: Comparison of endoscopic surgery, minimally invasive hematoma puncture, and external ventricular drainage. Chin J Traumatol. 2019. 22: 333-9
6. Gotoh S, Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N. Trends in the incidence and survival of intracerebral hemorrhage by its location in a Japanese community. Circ J. 2014. 78: 403-9
7. Goyal N, Tsivgoulis G, Malhotra K, Katsanos AH, Pandhi A, Alsherbini KA. Minimally invasive endoscopic hematoma evacuation vs best medical management for spontaneous basal-ganglia intracerebral hemorrhage. J Neurointerv Surg. 2019. 11: 579-83
8. Gregson BA, Mitchell P, Mendelow AD. Surgical decision making in brain hemorrhage. Stroke. 2019. 50: 1108-15
9. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019. 393: 1021-32
10. Hattori N, Katayama Y, Maya Y, Gatherer A. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: A randomized study. J Neurosurg. 2004. 101: 417-20
11. Hayashi T, Karibe H, Akamatsu Y, Narisawa A, Shoji T, Sasaki T. Endoscopic hematoma evacuation for intracerebral hemorrhage under local anesthesia: Factors that affect the hematoma removal rate. World Neurosurg. 2019. 126: e1330-6
12. Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001. 32: 891-7
13. Hersh EH, Gologorsky Y, Chartrain AG, Mocco J, Kellner CP. Minimally invasive surgery for intracerebral hemorrhage. Curr Neurol Neurosci Rep. 2018. 18: 34
14. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996. 27: 1304-5
15. Li L, Li Z, Li Y, Su R, Wang B, Gao L. Surgical evacuation of spontaneous cerebellar hemorrhage: Comparison of safety and efficacy of suboccipital craniotomy, stereotactic aspiration, and thrombolysis and endoscopic surgery. World Neurosurg. 2018. 117: e90-8
16. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): A randomised trial. Lancet. 2005. 365: 387-97
17. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet. 2013. 382: 397-408
18. Ogawa A.editors. Japanese Guideline for the Management of Stroke 2015. Tokyo, Japan: Kyowa Kikaku; 2015. p.
19. Qureshi A, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009. 373: 1632-44
20. Rennert RC, Tringale K, Steinberg JA, Warnke P, Konety I, Sand LA. Surgical management of spontaneous intracerebral hemorrhage: Insights from randomized controlled trials. Neurosurg Rev. 2020. 43: 999-1006
21. Song P, Duan FL, Cai Q, Wu JL, Chen XB, Wang Y. Endoscopic surgery versus external ventricular drainage surgery for severe intraventricular hemorrhage. Curr Med Sci. 2018. 38: 880-7
22. Sun G, Li X, Chen X, Zhang Y, Xu Z. Comparison of keyhole endoscopy and craniotomy for the treatment of patients with hypertensive cerebral hemorrhage. Medicine (Baltimore). 2019. 98: e14123
23. Xu X, Chen X, Li F, Zheng X, Wang Q, Sun G. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: A comparison with craniotomy. J Neurosurg. 2018. 128: 553-9
24. Yamamoto T, Esaki T, Nakao Y, Mori K. Endoscopic hematoma evacuation for the hypertensive putaminal hemorrhage. Jpn J Stroke. 2010. 32: 595-601
25. Yamamoto T, Nakao Y, Mori K, Maeda M. Endoscopic hematoma evacuation for hypertensive cerebellar hemorrhage. Minim Invasive Neurosurg. 2006. 49: 173-8
26. Yao Z, Hu X, You C, He M. Effect and feasibility of endoscopic surgery in spontaneous intracerebral hemorrhage: A systematic review and meta-analysis. World Neurosurg. 2018. 113: 348-56
27. Zhao YN, Chen XL. Endoscopic treatment of hypertensive intracerebral hemorrhage: A technical review. Chronic Dis Transl Med. 2016. 2: 140-6
28. Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: A meta-analysis of randomized controlled trials. Stroke. 2012. 43: 2923-30