- Department of Neurosurgery, Sree Chitra Tirunal Institute of Medical Science and Technology, Thiruvananthapuram, Kerala, India.
- Department of Pathology, Sree Chitra Tirunal Institute of Medical Science and Technology, Thiruvananthapuram, Kerala, India.
Correspondence Address:
H. V. Easwer, Department of Neurosurgery, Sree Chitra Tirunal Institute of Medical Science and Technology, Thiruvananthapuram, Kerala, India.
DOI:10.25259/SNI_1051_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: H. R. Darshan1, Biren Khimji Patel1, Ajit Singh1, Prakash Nair1, Rajalakshmi Poyuran2, H. V. Easwer1. Simultaneous trigonal and spinal meningioma with varied histology: A rare case report. 14-Dec-2021;12:611
How to cite this URL: H. R. Darshan1, Biren Khimji Patel1, Ajit Singh1, Prakash Nair1, Rajalakshmi Poyuran2, H. V. Easwer1. Simultaneous trigonal and spinal meningioma with varied histology: A rare case report. 14-Dec-2021;12:611. Available from: https://surgicalneurologyint.com/surgicalint-articles/11288/
Abstract
Background: Meningioma is one of the most common neoplasms of the central nervous system. Multiple meningiomas without neurofibromatosis are not a usual occurrence. Intraventricular meningioma with spinal meningioma is rare and not been reported in the literature.
Case Description: We report a case of a 63-year-old male with the left trigonal and spinal meningioma. Both the meningiomas were resected in different settings. The histological examination of tumors revealed to be of varied histology, that is, meningothelial and atypical meningioma, respectively.
Conclusion: Although various cases with multiple cranial and spinal meningiomas are described, this is the first case of an intraventricular and spinal meningioma. With varied histology, the case also reaffirms the theory of polyclonal origin of multiple meningiomas.
INTRODUCTION
Meningiomas are tumors of the covering of the brain. These are the most common non-glial primary brain tumors, which constitute almost 37% of all primary intracranial neoplasms and 53% of non-malignant tumors.[
CASE REPORT
A 63-year-old male presented complaints of weakness in bilateral lower limbs left more than right, paraesthesia in bilateral lower limbs and difficulty in walking from past 5 months. On examination, patient was found to have increased tone in bilateral lower limbs and power of 4/5(MRC grading) in bilateral lower limbs. Patient also had reduced touch and pain sensation below eighth thoracic dermatome level. Bladder function and sphincter control were preserved.
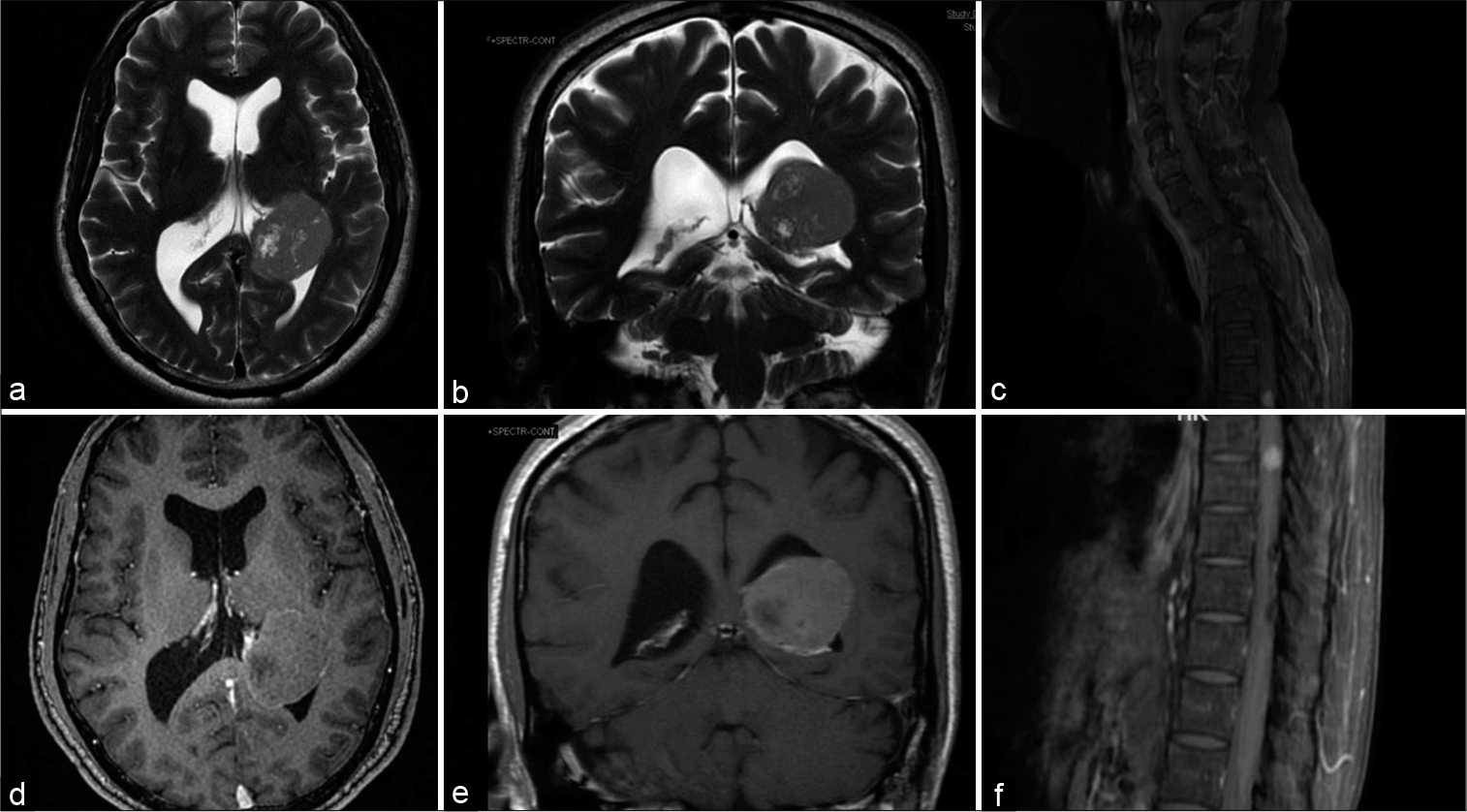
MRI demonstrated a T1 hypointense and T2 hyperintense and contrasts enhancing intradural extramedullary lesion at eighth thoracic level. Contrast MRI of the brain revealed a 4.2 × 3.6 × 3.5 cm lesion in the trigonal area of the left lateral ventricle. The lesion was T1 hypointense and heterogeneously contrasts enhancing [
Patient, due to his symptoms of the spinal lesion underwent excision of the spinal lesion. D7 and D8 laminectomy was performed. Tumor was dorsal placed and total excision along with attached dura was performed. Dural defect was closed and wound closed with a drain in situ. Patient’s spasticity and gait improved in the postoperative period. Patient complained of headache in the postoperative period and hence MRI brain was performed. The cranial lesion was planned for a second stage surgery since patient did not exhibit any symptom for the same.
On follow-up patient presented to us with two episodes of seizures, 2 months after the spinal surgery and patient was admitted for excision of the cranial component. The left parietal craniotomy was performed and trigone was approached through the keens point. Excision was performed and patient had an uneventful postoperative course.
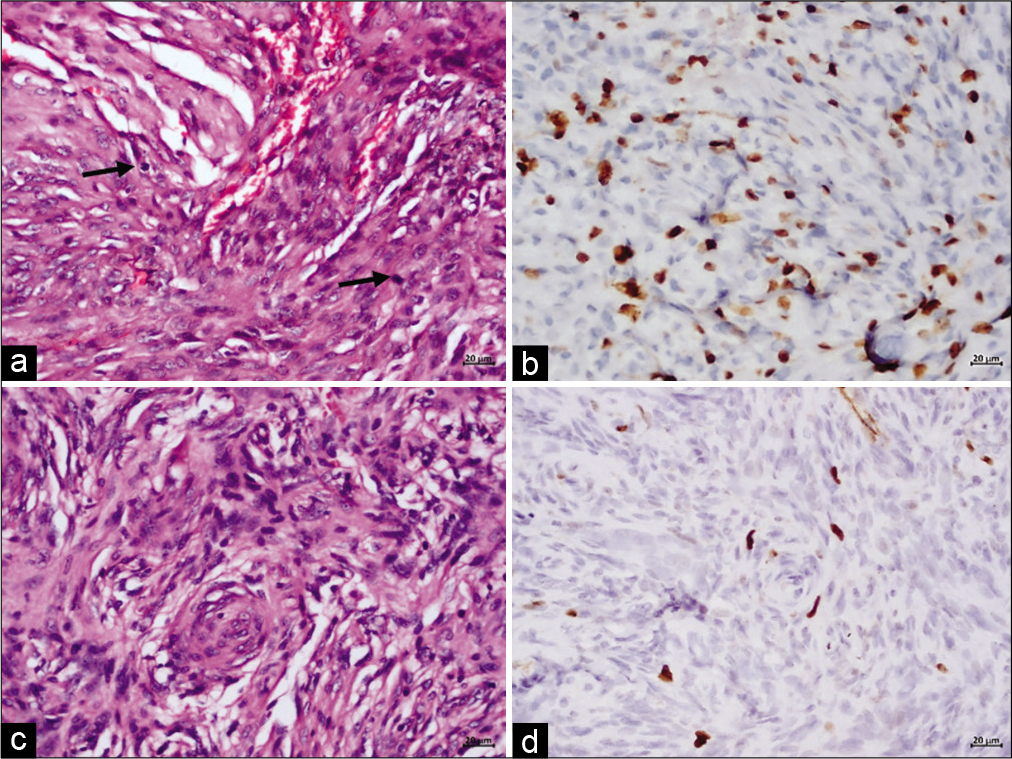
Microscopic examination of the spinal tumor showed a meningothelial neoplasm having transitional morphology. The cells were moderately pleomorphic and were arranged in fascicles, whorls, and lobules with few psammoma bodies. The tumor had mitotic activity reaching upto 4/10hpf and a Ki-67 labeling index of 12–15%. Due to the increased mitotic count, this tumor was classified as atypical meningioma, the WHO Grade II [
Figure 2:
Meningioma excised from D8 spinal level showing features of atypical meningioma, WHO Grade II with increased mitotic activity (a, mitotic figures marked by arrows) and elevated Ki-67 labelling index. In contrast, the neoplasm excised from trigone exhibits morphological features of transitional meningioma, WHO Grade I with low Ki-67 labelling index (d). (a and c) Haematoxylin and eosin; (b and d) Immunoperoxidase; Magnification=scale bar (a-d: 20 μm)
Resected tissue from the trigone showed a meningothelial neoplasm exhibiting transitional morphology with few psammoma bodies, lymphocytic, and foamy macrophage infiltration. The cells were arranged in fascicles and whorls and had moderate nuclear pleomorphism, mitotic activity of 1–2/10hpf and Ki-67 labeling index of 4–5%. There was no evidence of brain parenchymal invasion. These features were of a transitional meningioma, the WHO Grade I.
DISCUSSION
Anifimow and Blumenau were the first to report multiple meningiomas as early as 1889.[
Meningiomas are called multiple when they occur as at least two in the neuraxis spatially separated and at the same time or more than two arising sequentially from two distinct regions.[
Meningiomas are a frequent occurrence in NF2 found in about half of the patients. NF2 is usually the disease in young people when they have multiple meningiomas. However, adults with multiple meningiomas but no vestibular schwannomas are highly unlikely to be affected by neurofibromatosis 2.[
Intraventricular meningiomas are a rare variant and constitute of only about 0.5–5% of all intracranial meningiomas.[
Pathogenesis of multiple meningiomas has been tried to explain in two theories. The two theories are: (1) either these arise independently as proven by differences seen in the histological or cytogenic differences between the tumors themselves. (2) One transforming event and clone cells spread throughout the meninges leading to the development of clonally related multiple tumors.
Female preponderance is a usual observation in meningiomas. This stands true in case of intraventricular meningiomas, that is, 2:1[
Operative management of multiple meningiomas has remained unsettled for a long period now. Which tumor to operated first and when to operate has posed a serious dilemma? Not all tumors in case of multiple meningiomas need to be operated. Resection of multiple intracranial and spinal meningiomas has been reported. Intraventricular meningiomas are very different in the way of management than their supratentorial counterparts.
Atypical meningiomas have a recurrence rate of 29–38% of cases.[
The majority of multiple meningiomas described in the literature are benign. Moreover, in case of spinal multiple meningiomas psammomatous variant predominates. Histologically, case described by Sedzimir et al.[
Thus, reaffirming one of the theories of the pathogenesis stated that these tumors arise independently and are clonally different tumors. Elderly age, male sex, and histology make our case a unique one in the literature.
CONCLUSION
Multiple meningiomas along the neuraxis and intraventricular meningiomas are both rare entities. Multiple meningiomas in the absence of NF2 are a rare entity but should be kept in mind always in case of varied clinical manifestations. Two histological variants of meningioma in cranium and spine are a proof of the origin of meningioma through polyclonal cells. Atypical meningiomas (the WHO Grade 2) are extremely rare in spinal canal and will warrant a complete resection and close follow-up.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anfimow J, Blumenau L. Ein Fall multiplier Geschwulste in der Schadelhohle. Neurol Zentralbl. 1889. 8: 585
2. Bhatoe HS. Simultaneous occurrence of multiple meningiomas in different neuraxial compartments. Neurol India. 2003. 51: 263-5
3. Butti G, Assietti R, Casalone R, Paoletti . Multiple meningiomas. A clinical surgical and cytogenetic analysis. Surg Neurol. 1989. 31: 255-60
4. Capodano AM. Nervous system tumors: Meningioma. Atlas Genet Cytogenet Oncol Haematol. 2000. 4: 250-3
5. Erman T, Göcer AI, Erdogan E, Boyar B, Hacyakupoglu S, Zorludemir S. Intraventricular meningiomas: A review and report of eight cases. Neurosurg Q. 2004. 14: 154-60
6. Evans DG, Watson C, King A, Wallace AJ, Baser ME. Multiple meningiomas: Differential involvement of the NF2 gene in children and adults J Med Genet. 2005. 42: 45-8
7. Gezen F, Kahraman S, Canakci Z, Beduk A. Review of 36 cases of spinal meningioma. Spine. 2000. 25: 727-31
8. Guidetti B, Delfini R, Al-Mefty O.editors. Lateral and fourth ventricle meningiomas. Meningiomas. New York: Raven Press; 1991. p. 569-87
9. Liu M, Wei y, Liu Y, Zhu S, Li X. Intraventricular meninigiomas: A report of 25 cases. Neurosurg Rev. 2006. 29: 36-40
10. Menon G, Nair S, Sudhir J, Rao R, Easwer HV, Krishnakumar K. Meningiomas of the lateral ventricle a report of 15 cases. Br J Neurosurg. 2009. 23: 297-303
11. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019. 21: v1-100
12. Roux FX, Nataf F, Pinaudeau M, Borne G, Deveaux B, Meder JF. Intraspinal meningiomas: Review of 54 cases with discussion of poor prognosis, factors and modern therapeutic management. Surg Neurol. 1996. 46: 458-64
13. Sedzimir CB, Frazer AK, Roberts JR. Cranial and spinal meningioma in a pair of twin boys. J Neurol Neurosurg Psych. 1973. 35: 368
14. Spallone A, Neroni M, Giuffre R. Multiple skull base meningiomas: Case report. Surg Neurol. 1999. 51: 274-80
15. Yoon SH, Chung CK, Jahng TA. Surgical outcome of spinal canal meningiomas. J Korean Neurosurg Soc. 2007. 42: 300-4