- Department of Neurosurgery, University of Iowa Hospitals and Clinics, Iowa City, United States.
DOI:10.25259/SNI_767_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Scott Christopher Seaman, Muhammad Salman Ali, Anthony Marincovich, Carlos Osorno-Cruz, Jeremy D. W. Greenlee. Single surgeon experience with minimally invasive supraorbital craniotomy versus bifrontal craniotomy for anterior skull base meningiomas. 22-Dec-2020;11:458
How to cite this URL: Scott Christopher Seaman, Muhammad Salman Ali, Anthony Marincovich, Carlos Osorno-Cruz, Jeremy D. W. Greenlee. Single surgeon experience with minimally invasive supraorbital craniotomy versus bifrontal craniotomy for anterior skull base meningiomas. 22-Dec-2020;11:458. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10483
Abstract
Background: Anterior skull base meningiomas (ASBMs) account for about 10% of meningiomas. Bifrontal craniotomy (BFC) represents the traditional transcranial approach to accessing meningiomas in these locations. Supraorbital craniotomy (SOC) provides a minimally invasive subfrontal corridor in select patients. Here, we present our series of ASBM accessed by SOC and BFC by a single surgeon to review decision-making and compare outcomes in both techniques.
Methods: Thirty-three patients were identified with ASBM. Age, tumor characteristics, presenting symptoms, postoperative complications, and outcomes were analyzed.
Results: Bifrontal and SOC were performed in 13 and 20 patients, respectively. Mean follow-up time was 98.4 months. Patients undergoing SOC had smaller tumor size, located farther from the posterior table of frontal sinus, had less peritumoral edema, and decreased length of stay compared to patients undergoing BFC. Extent of resection was slightly better with BFC (99.8%) compared to SOC (91.8%), although this difference did not reach statistical significance. Recurrence-free survival and rate of re-do surgeries were not different between two groups. BFC was associated with higher rates of postoperative encephalomalacia.
Conclusion: SOC provides an excellent surgical option for ASBMs providing comparable extent of resection, minimal manipulation of brain, and excellent cosmetic outcomes for patients. The patient selection is key to maximize the benefits from this approach.
Keywords: Anterior skull base case series, Bifrontal craniotomy, Keyhole approaches, Meningioma, Supraorbital craniotomy
INTRODUCTION
Meningiomas are the most common primary brain tumor.[
The supraorbital craniotomy (SOC) keyhole approach mitigates many of the unwanted results of large standard craniotomies for access to frontal or subfrontal pathology. Since its development by Krause, it has been further refined and its use expanded.[
To assess the application of the supraorbital approach in ASBM and compare it to a traditional subfrontal corridor, we retrospectively reviewed our database and found 33 cases of meningiomas in the anterior skull base resected using either bifrontal craniotomy (BFC) or SOC performed by a single surgeon. We then describe our approach selection criteria with the SOC and BFC.
MATERIALS AND METHODS
Patient selection
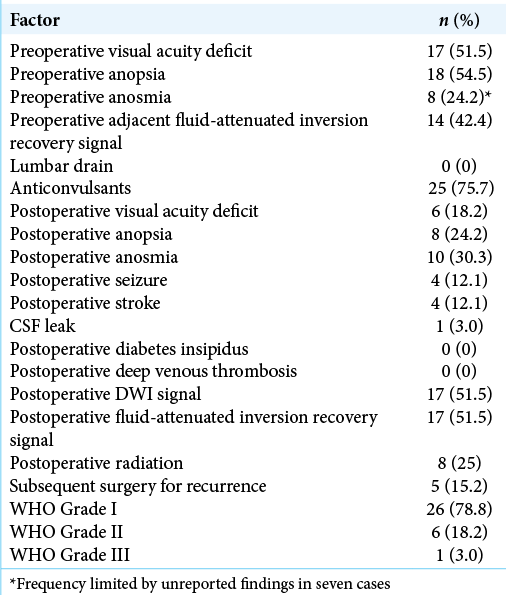
A retrospective chart review from January 2005 to January 2020 with appropriate IRB approval was performed. All patients included had procedures performed by the senior author (JDG) and selection criteria included a diagnosis of meningioma and procedural codes consistent with a SOC with or without endoscopic assistance or a bilateral craniotomy. For the BFC group, the preoperative images and operative report were examined to confirm an ASBM and BFC was utilized given the lack of specificity with the diagnostic codes. Basic demographic factors (age, gender, body mass index, symptomology [visual acuity/anopsia deficits], and anosmia), radiographic factors (tumor volume assessed by [A*B*C]/2 measurements, tumor location, abnormal adjacent fluid-attenuated inversion recovery [FLAIR] signal to assess for brain adherence and/or infiltration, as well as encephalomalacia, distance from posterior wall of frontal bone), operative features (use of lumbar drain, anticonvulsants), and postoperative outcomes (extent of resection, cerebrospinal fluid [CSF] leak, recurrence requiring subsequent surgery, postoperative radiation, length of stay, deep venous thrombosis, pulmonary embolism, stroke, seizure, and diabetes insipidus) were collected. Final pathology and time to recurrence were also recorded. In addition, the presence of encephalomalacia on the most recent available follow-up scan was documented.
Statistical analysis
Statistical analysis was conducted with IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA). Continuous variables were compared using a Student’s t-test or Welch’s t-test if Levene’s assumption for normality was violated. Categorical variables were compared using a Chi-square test or Fisher’s test in cases where one of the matrix groups had a sample less than 5. Kaplan–Meir curves for recurrence between approaches were calculated using the Mantel-Cox log-rank test. Two-way ANOVA with post hoc between group analyses with Bonferroni correction for multiple comparisons was used to assess the extent of resection by approach and tumor recurrence necessitating repeat operation.
RESULTS
Demographics and clinical presentation
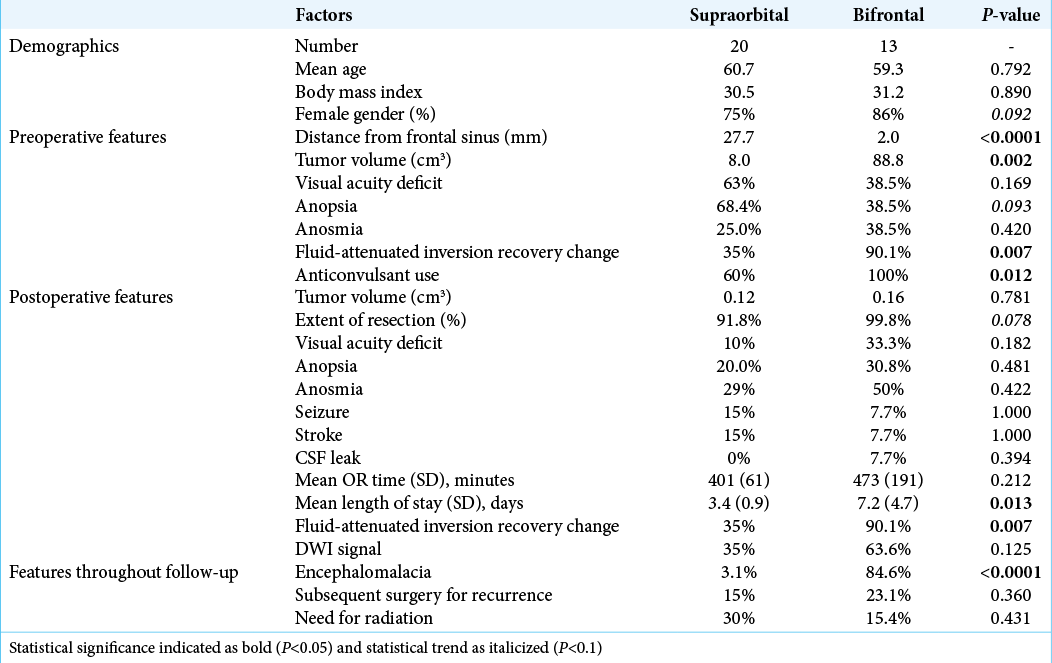
There were 33 total patients identified, 20 of which were approached through a minimally invasive SOC and 13 approached through a BFC approach. The overall demographics are reported in [
Differences between approaches
Comparing approaches, there was no difference in demographic features or preoperative neurologic deficits [
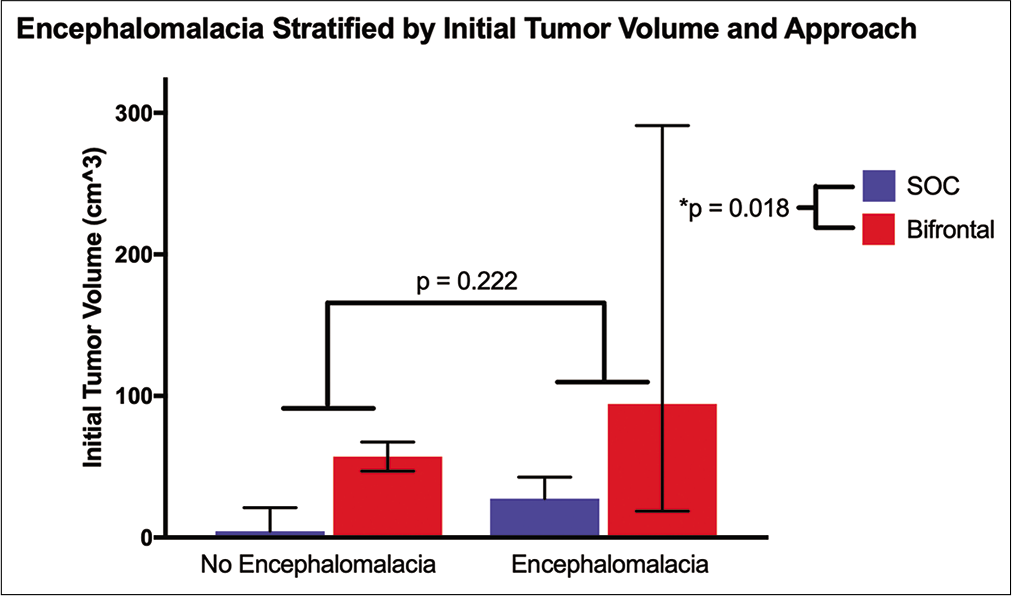
Association with encephalomalacia
Meningiomas approached with a BFC had more encephalomalacia at last follow-up (84.6% vs. 3.1%, P < 0.0001). To evaluate if there was any effect of approach versus initial tumor volume on encephalomalacia, a two-way ANOVA was performed. The mean volume in the BFC without encephalomalacia was 57.3 cm3 (range 47.1–67.6) compared to 94.5 cm3 (range 18.7–291.2) in those who developed encephalomalacia. In the SOC group, those who never developed encephalomalacia had a mean initial volume of 4.6 cm3 (range 0.61–21.2) compared to 27.3 cm3 (range 7.9–42.6) in those who did develop encephalomalacia [
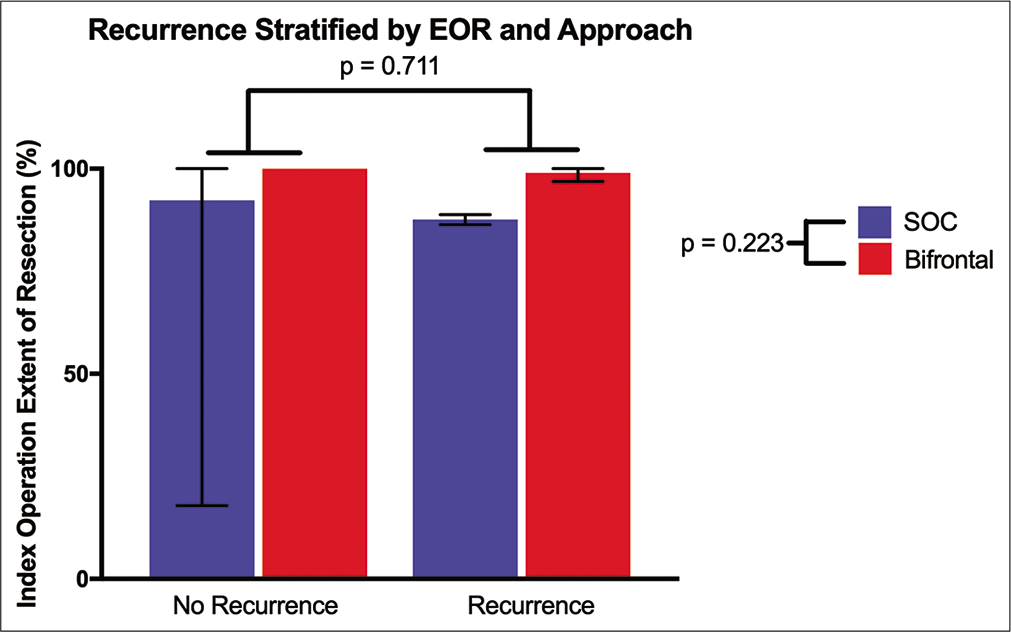
Recurrent cases requiring intervention
Overall, there was no difference between approaches that required subsequent surgery (P = 0.360). In addition, Mantel-Cox log-rank test showed no difference in recurrence free survival (P = 0.102). Two-way ANOVA testing was performed evaluating recurrent tumors by approach and extent of resection. Single BFC had a mean EOR of 100% versus 98.9% in recurrent BFC cases. In the SOC group, the single surgery cohort had a mean EOR of 92.3% versus 87.6% in the recurrent surgery cohort. There was no difference in EOR by approach (P = 0.223) or repeat resection status (P = 0.711) [
Figure 2:
Recurrent tumor analysis evaluating effect of surgical approach and index surgery extent of resection represented as mean (error bars: range). There was no significant difference, indicating the minimally invasive supraorbital craniotomy is an effective option in small- to medium-sized meningiomas.
DISCUSSION
This study shows a single surgeon’s experience using minimally invasive SOC is not inferior to standard BFC for midline ASBM. In particular, there was no difference in extent of resection, recurrence, or postoperative neurologic deficits between groups. Moreover, SOC had less incidence of encephalomalacia at follow-up compared to BFC. SOC had shorter operative times on average by 1 h and a length of stay that was significantly shorter than BFC.
Minimizing encephalomalacia
In our study, patients undergoing BFC have higher rate of postoperative encephalomalacia noted on magnetic resonance imaging on last clinic visit compared to SOC. Although postoperative FLAIR signal is higher for BFC, the patients undergoing this approach have much larger tumor size. For the SOC group, those who developed encephalomalacia had a mean volume of 27.3 cm3 compared to 94.5 cm3 in the BFC group. We hypothesized that initial tumor volume primarily contributes to the formation of encephalomalacia postoperatively; however, the mean volume in the BFC group that did not develop encephalomalacia was 57.3 cm3, far larger than the volume in SOC cases that did develop encephalomalacia at 27.3cm3. This suggests that tumor volume alone does not contribute to the formation of encephalomalacia and implicates the effects of retraction, as the amount of retraction necessary to remove a 4.6 cm3 tumor through SOC approach differs from a 27.3 cm3 tumor removal.
Perneczky reported in their 10-year experience with SOC that a majority of the meningiomas resected with this approach were between 2.5 and 4.4 cm and gross total resection was achieved in 89% of the cases.[
Minimizing length of stay and use of hospital resources
We found that SOC affords decreased operative time and length of hospital stay compared to BFC. Although the difference in operative time between the two groups was not significant, this is likely limited by our small sample size, as the procedural time was on average shorter by 1 h. Length of stay was shorter by nearly 4 days in our series. Short procedure time and length of stay decrease resource utilization and maximize hospital throughput. In addition, shorter procedure times and length of stay may contribute to better outcomes in elderly patients with meningiomas. As the incidence of meningiomas increases significantly with age,[
Defining tumor characteristics for approach selection
We have reported slightly higher extent of resection with BFC 99.8% versus 91.8% for SOC but did not find any significant difference in recurrence free survival and the need for reoperation for recurrence between the two groups. As we have demonstrated in our series, patients undergoing SOC usually are located more posteriorly in the anterior skull base and involve PS and TS, have a higher percentage of those who are presenting with visual deficits, and are usually smaller in size compared to larger meningiomas found in OG. The goal of surgery for the lesions in PS and TS is improvement of visual symptoms and maximal safe resection of the tumor [
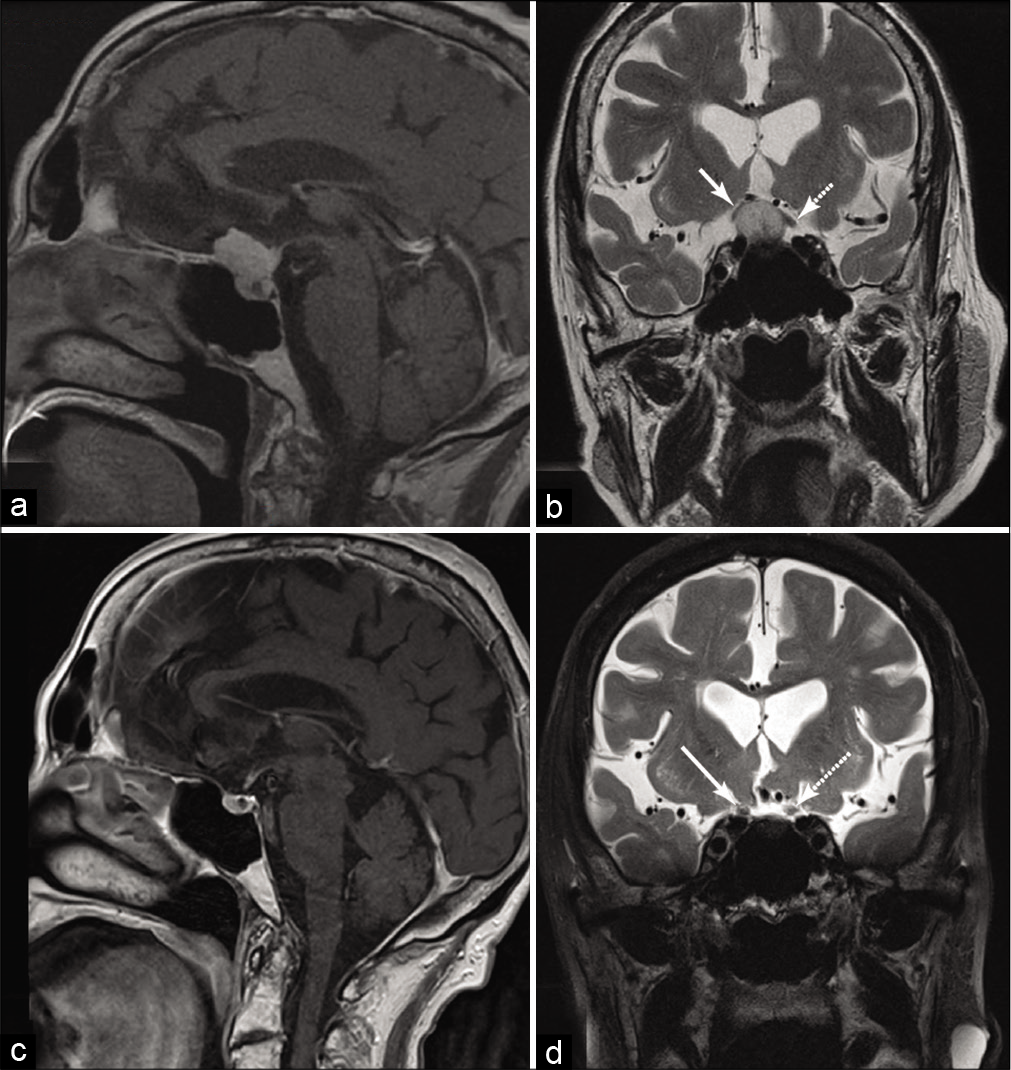
Figure 3:
Sagittal postgadolinium-enhanced T1 magnetic resonance imaging (a) depicting a planum/tuberculum meningioma with coronal T2, (b) depicting elevation of the optic nerves bilaterally, right (solid arrow) greater than left (dashed arrow) causing peripheral vision loss. Postoperatively, her vision improved and 3 years after right eyebrow supraorbital craniotomy, sagittal T1 enhanced magnetic resonance imaging shows satisfactory gross total resection (c) with coronal T2 (d) showing resolution of the displaced optic nerves (solid arrow right, dashed arrow left).
Regardless of slightly better extent of resection, BFC carries with it significant surgical complications. Morbidity reported from BFC for OG meningiomas has been reported to occur in as much of 30%, mainly consisting of hydrocephalus necessitating temporary or permanent CSF diversion, seizures, CSF fistula, or wound infections,[
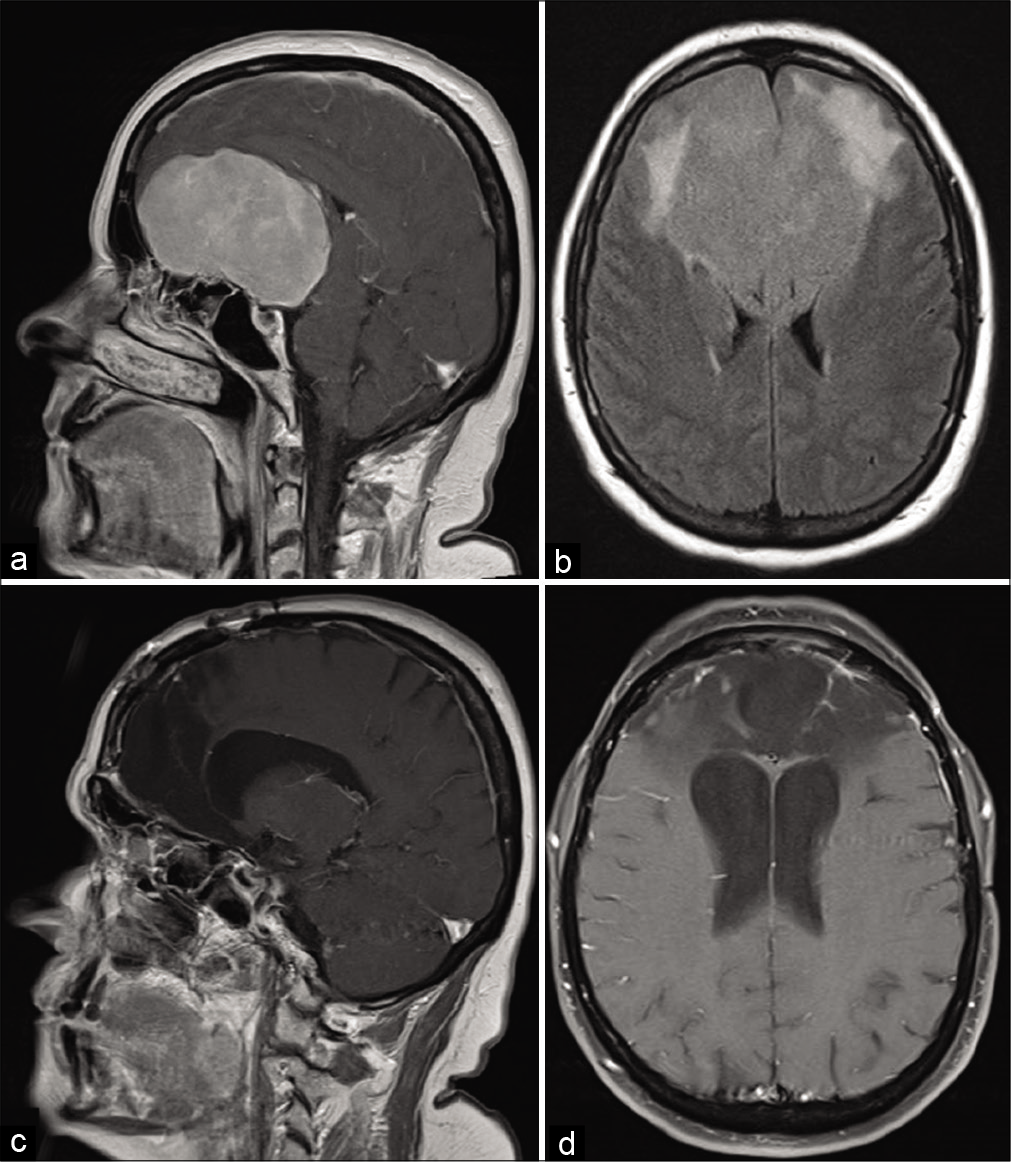
Figure 4:
Sagittal postgadolinium-enhanced T1 magnetic resonance imaging depicting a large olfactory groove meningioma spanning the entirety of the anterior skull base from posterior table of the frontal sinuses to suprasellar region (a) with significant mass effect on the lateral horns of the ventricles and associated fluid-attenuated inversion recovery signal change. (b) Eight months after bifrontal craniotomy and tumor resection, sagittal (c) and axial (d) postcontrast views show gross total resection with associated encephalomalacia.
Limitations of the SOC
One of the criteria we follow in our decision-making is the tumor size and peritumoral FLAIR signal. As the tumor grows vertically from the anterior skull base, it presents a limitation for SOC, as the surgical corridor in SOC is mostly horizontal; this limitation can be overcome to a degree with the inclusion of an orbital osteotomy with the SOC as a minimally invasive version of the modified orbitozygomatic osteotomy.[
A recent cadaveric study has suggested limited ability to drill the inferior crista galli and cribriform plate with SOC.[
We have not noticed any muscle wasting and excellent wound healing in our series, although a formal comparison between approaches was limited by our observations primarily in clinic evaluation and not in a standardized approach. Increased meningioma incidence among elderly population is also a concern for men with baldness. Cosmetic results have been linked with patient outcomes.[
CONCLUSION
Supraorbital approach provides an excellent corridor to access meningioma in the anterior skull base. We have addressed some of the issues historically linked with SOC, that is, extent of resection, neurovascular injury, tumor recurrence, and requirement of re-do surgery. It provides a safe passage for tumor resection, without injury to brain and neurovascular structures, and excellent cosmetic results in carefully selected patients. As we tailor approaches to each patient according to their own individual needs, mastering SOC, BFC, and EEA approaches and their associated technical nuances and patient selection criteria will allow us to provide a comprehensive approach to our patients.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abbassy M, Woodard TD, Sindwani R, Recinos PF. An overview of anterior skull base meningiomas and the endoscopic endonasal approach. Otolaryngol Clin North Am. 2016. 49: 141-52
2. Almefty GF, Al-Mefty O.editors. Meningioma. Youmans and Winn Neurological Surgery. Amsterdam: Elsevier; 2016. p. 1107-32
3. Andrews RJ, Bringas JR. A review of brain retraction and recommendations for minimizing intraoperative brain injury. Neurosurgery. 1993. 33: 1052-63
4. Banu MA, Mehta A, Ottenhausen M, Fraser JF, Patel KS, Szentirmai O. Endoscope-assisted endonasal versus supraorbital keyhole resection of olfactory groove meningiomas: Comparison and combination of 2 minimally invasive approaches. J Neurosurg. 2016. 124: 605-20
5. Borghei-Razavi H, Truong HQ, Fernandes-Cabral DT, Celtikci E, Chabot JD, Stefko ST. Minimally invasive approaches for anterior skull base meningiomas: Supraorbital eyebrow, endoscopic endonasal, or a combination of both? Anatomic study, limitations, and surgical application. World Neurosurg. 2018. 112: e666-74
6. Chamoun R, Krisht KM, Couldwell WT. Incidental meningiomas. Neurosurg Focus. 2011. 31: E19
7. Cohen AR, Perneczky A, Rodziewicz GS, Gingold SI. Endoscope-assisted craniotomy: Approach to the rostral brain stem. Neurosurgery. 1995. 36: 1128-9
8. Dlouhy BJ, Chae MP, Teo C. The supraorbital eyebrow approach in children: Clinical outcomes, cosmetic results, and complications. J Neurosurg Pediatr. 2015. 15: 12-9
9. Gardner PA, Kassam AB, Thomas A, Snyderman CH, Carrau RL, Mintz AH. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008. 63: 36-52
10. Iacoangeli M, Nocchi N, Nasi D, di Rienzo A, Dobran M, Gladi M. Minimally invasive supraorbital keyhole approach for the treatment of anterior cranial fossa meningiomas. Neurol Med Chir (Tokyo). 2016. 56: 180-5
11. Nakamura M, Struck M, Roser F, Vorkapic P, Samii M. Olfactory groove meningiomas: Clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007. 60: 844-52
12. Ottenhausen M, Rumalla K, Alalade AF, Nair P, La Corte E, Younus I. Decision-making algorithm for minimally invasive approaches to anterior skull base meningiomas. Neurosurg Focus. 2018. 44: E7
13. Perneczky A, Reisch R.editors. Basal variation of the supraorbital approach: The supraorbito-orbital craniotomy. Keyhole Approaches in Neurosurgery, Concept and Surgical Technique. Lavanttal, Austria: Springer; 2008. 1: 83-97
14. Reisch R, Marcus HJ, Hugelshofer M, Koechlin NO, Stadie A, Kockro RA. Patients’ cosmetic satisfaction, pain, and functional outcomes after supraorbital craniotomy through an eyebrow incision. J Neurosurg. 2014. 121: 730-4
15. Reisch R, Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005. 57: 242-55
16. Schwartz TH. An eyebrow for an eyebrow and a nose for a nose. World Neurosurg. 2014. 82: e97-9
17. Spetzler RF, Sanai N. The quiet revolution: Retractorless surgery for complex vascular and skull base lesions. J Neurosurg. 2012. 116: 291-300
18. Yaşargil M.editors. Microneurosurgery. New York: Georg Thieme Verlag; 1984. p.
19. Zoli M, Guaraldi F, Pasquini E, Frank G, Mazzatenta D. The endoscopic endonasal management of anterior skull base meningiomas. J Neurol Surg B Skull Base. 2018. 79: S300-10