- Department of Neurosurgery, University Hospital Puerta de Hierro Majadahonda, Majadahonda, Madrid, Spain.
- Department of Radiology, University Hospital Puerta de Hierro Majadahonda, Majadahonda, Madrid, Spain.
Correspondence Address:
Xavier Santander, Department of Neurosurgery, University Hospital Puerta de Hierro Majadahonda, Majadahonda, Madrid, Spain.
DOI:10.25259/SNI_582_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Xavier Santander1, Yolanda García Hidalgo2, José Carlos Flores2, Blanca Gómez-Jordana1. Sinking skin syndrome in a decompressive craniectomy series: Clinical and radiological features. 16-Sep-2022;13:422
How to cite this URL: Xavier Santander1, Yolanda García Hidalgo2, José Carlos Flores2, Blanca Gómez-Jordana1. Sinking skin syndrome in a decompressive craniectomy series: Clinical and radiological features. 16-Sep-2022;13:422. Available from: https://surgicalneurologyint.com/surgicalint-articles/11869/
Abstract
Background: The sinking skin syndrome (SSS) is a particular complication after a decompressive craniectomy (DC). It still remains a poorly understood and underestimated entity.
Methods: Retrospective case series of craniectomized patients with and without SSS. Clinical and radiological features (DC diameter, shape of craniectomy flap, and midline deviation) were described and relative volumes of intracranial loss were quantified.
Results: Twenty-seven patients (63% with SSS). The most common indication for DC was traumatic brain injury: 48.15%. The p50 diameter of DC was 12.8 cm for patients with SSS and 11.1 cm for patients without (Z score = 0.32). DC area was 81.5 cm2 for patients with SSS and 71.43 cm2 for patients without the syndrome (Z score = 0.61). According to the shape of the craniectomy flap, we classified our patients as: «same level» (51.8%), «sunken» (25.9%), and «extracranial herniation» (14.8%). Two patients (7.4%) had paradoxical herniation. Midline deviation was present in 12 (70.6%) patients with SSS. The 3rd ventricle volume average was 1.2 cc for patients with SSS versus 2.35 cc for patients without (Z score = 0.04). About 94.11% of patients (16 out of 17) clearly improved after replacement of the cranial defect.
Conclusion: In our series, low 3rd ventricle volumes had a good relation with SSS. The presence of a sunken flap does not guarantee SSS per se and we propose the following radiologic description: A = sunken, B = same level, C = extracranial herniation, and D = paradoxical. Replacement of the skull defect is the main treatment.
Keywords: Craniectomy, Decompressive, Paradoxical herniation, Sinking flap, Sinking skin syndrome
INTRODUCTION
The sinking skin syndrome (SSS) or syndrome of the trephined, as first described by Grant and Norcross,[
Based on an array of case series, many clinical features of this syndrome are described in the literature.[
To date, many mechanisms have been lucubrated to explain the pathophysiology of this phenomenon. Some authors have suggested brain pulsation due to arterial and venous pressure variations,[
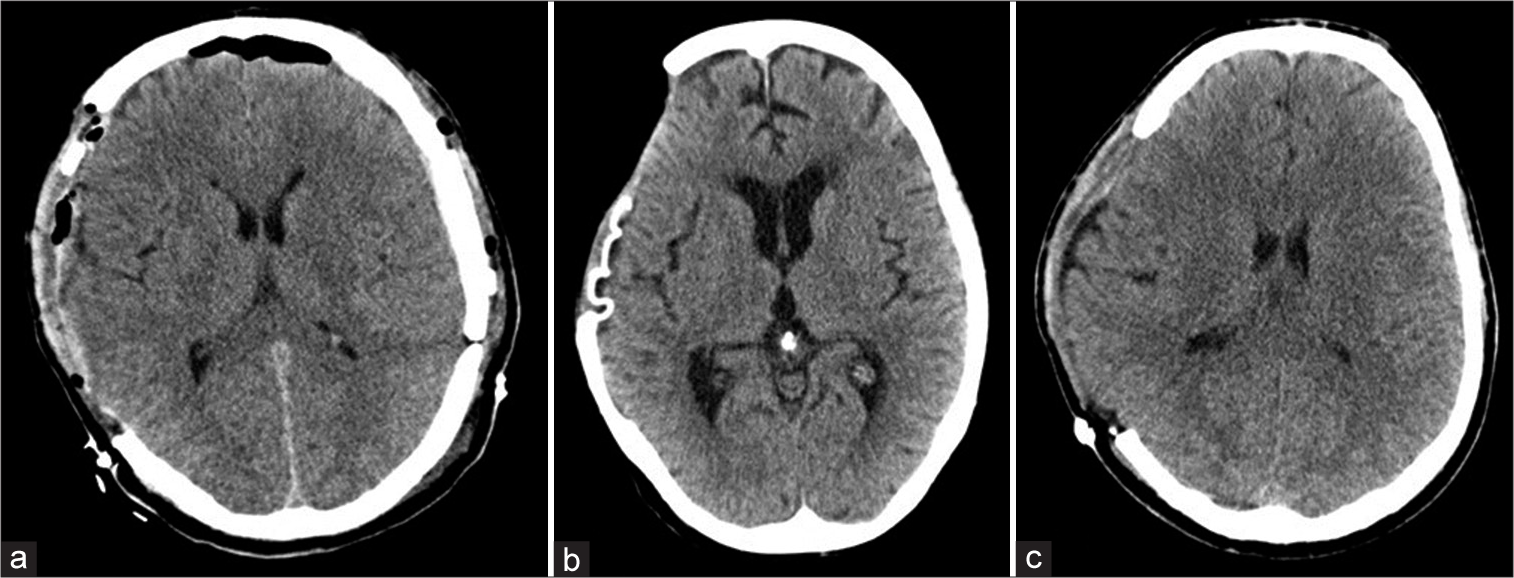
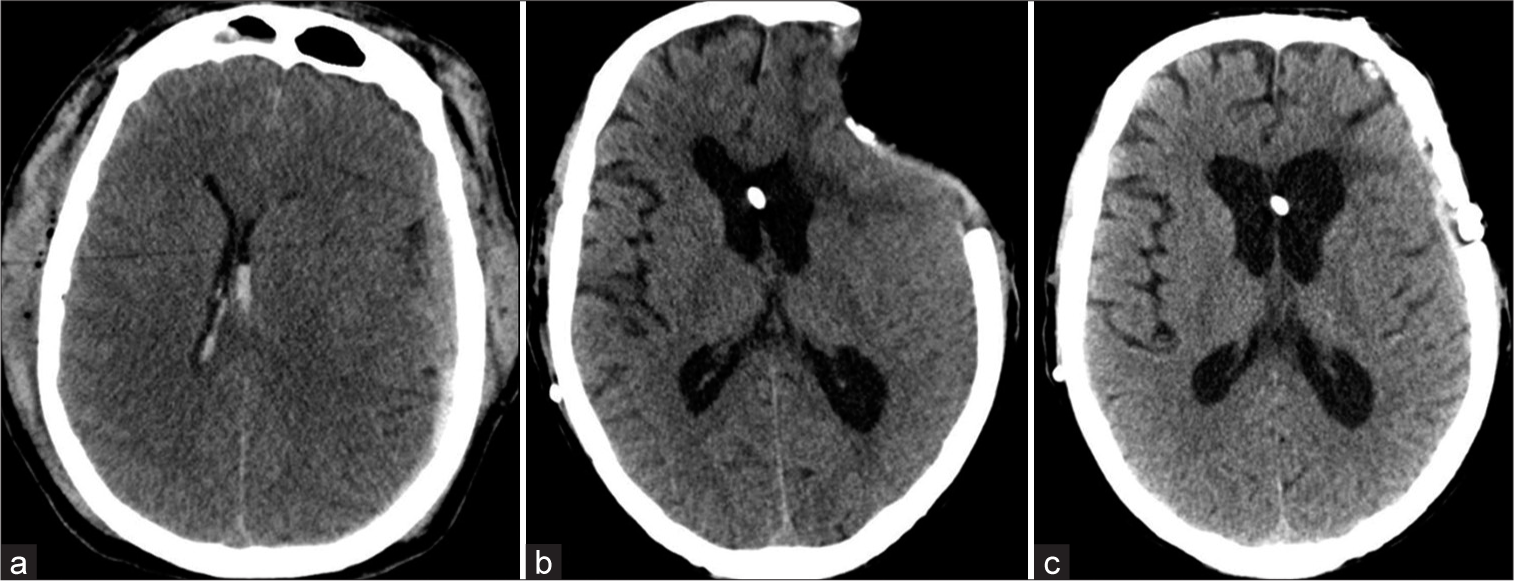
From the radiological point of view, the brain of a postcraniectomy patient can undergo one of three following scenarios: (1) the brain is at the same level of the craniectomy flap, (2) the brain is sunken (sunken flap sign), or (3) it can be herniated extracranially. Sometimes, in the context of a sunken brain, a contralateral deviation of the midline can occur giving place a complication called paradoxical herniation. To complicate things further, the SSS can be observed in patients without the sunken flap sign (i.e., extracranial herniation) suggesting that the SSS could be a misnomer.[
The main treatment, as mentioned before, is the cranioplasty.[
MATERIALS AND METHODS
During the period of 2010–2016, we reviewed retrospectively cases of patients who underwent DC. We collected epidemiological data and images. Two groups were conformed according to the presence or absence of SSS. Initial diagnostic prompting the DC, area, and diameter was described in both groups. In the group of patients with SSS, we also described clinical symptoms and average time from DC to the beginning of symptoms, average time from DC to cranioplasty, and whether they improved afterward.
Radiologically, we described the shape of the craniectomy flap as sunken, in the same level and extracranial herniation. Midline deviation below 5 mm and the presence of paradoxical herniation (defined as deviation of the midline by more than 5 mm away from the craniectomy site) are also described [
For the statistical analysis, we used Fisher’s exact test for categorical variables and using Mann–Whitney’s Student’s t-test or U-test on numerical ones. The significance level has been set to 0.05 and the statistical package used was Stata V.16 (StataCorp LLC.).
RESULTS
Through search in our data banks, 98 cases of patients who underwent DC were identified between the 2010 and 2016 period. Of these patients, only 27 had all the relevant clinical information mentioned lines above to conform our series. A graphic describing the process of inclusion/exclusion criteria is shown in
Age and sex
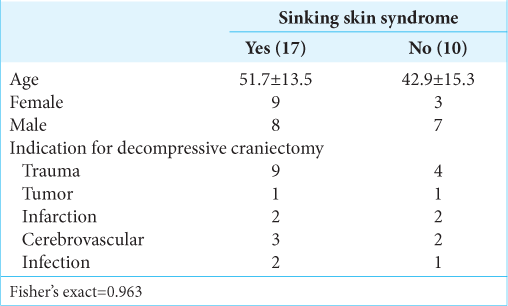
The mean age in our series was 48.4 years. In the group of patients with SSS, the median age was 51.7 years, and 53% were female and 47% were male. In the group corresponding to patients without the syndrome, the mean age was 42.9 years and 30% were female and 70% were male [z score = 0.4,
Indications for DC
The most common indication for DC, in general, was traumatic brain injury (TBI) accounting for 48.15% (13 patients), followed by hemorrhagic causes in 18.5% (five patients), ischemic in 14.8%, infection in 11.1%, and tumor in 7.4%. TBI was also the main cause for DC in the group of patients with SSS and the group of patients without the syndrome (53% and 40%, respectively) [
Size and characteristics of DC
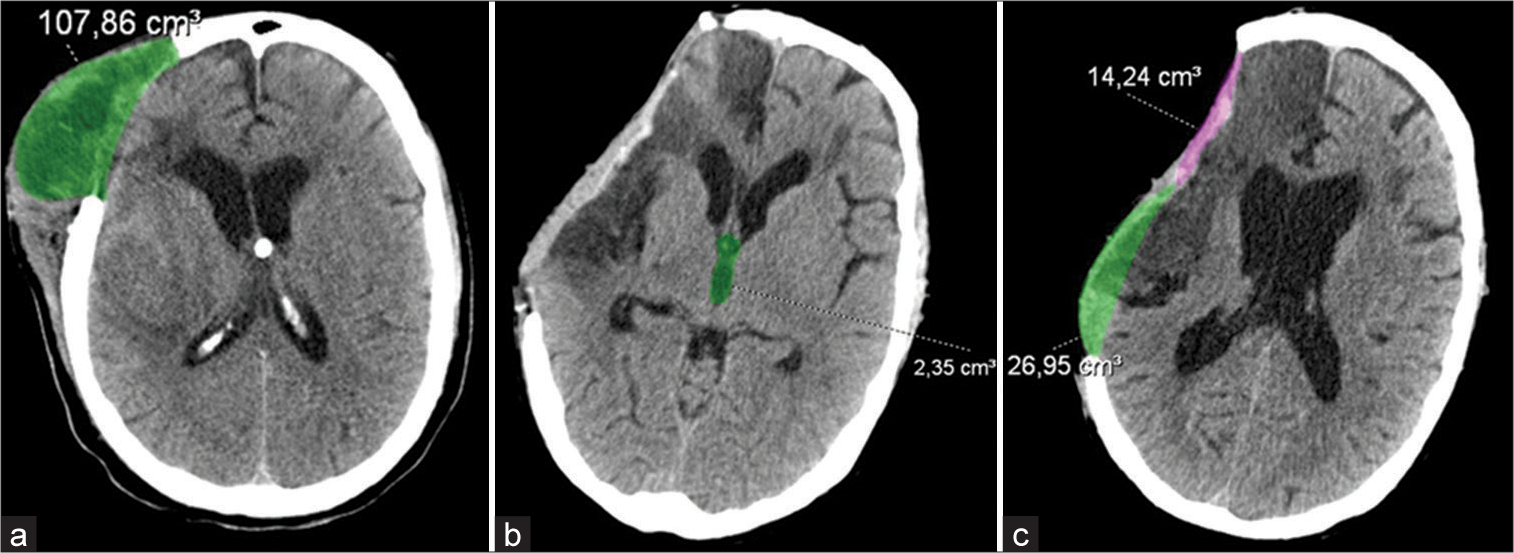
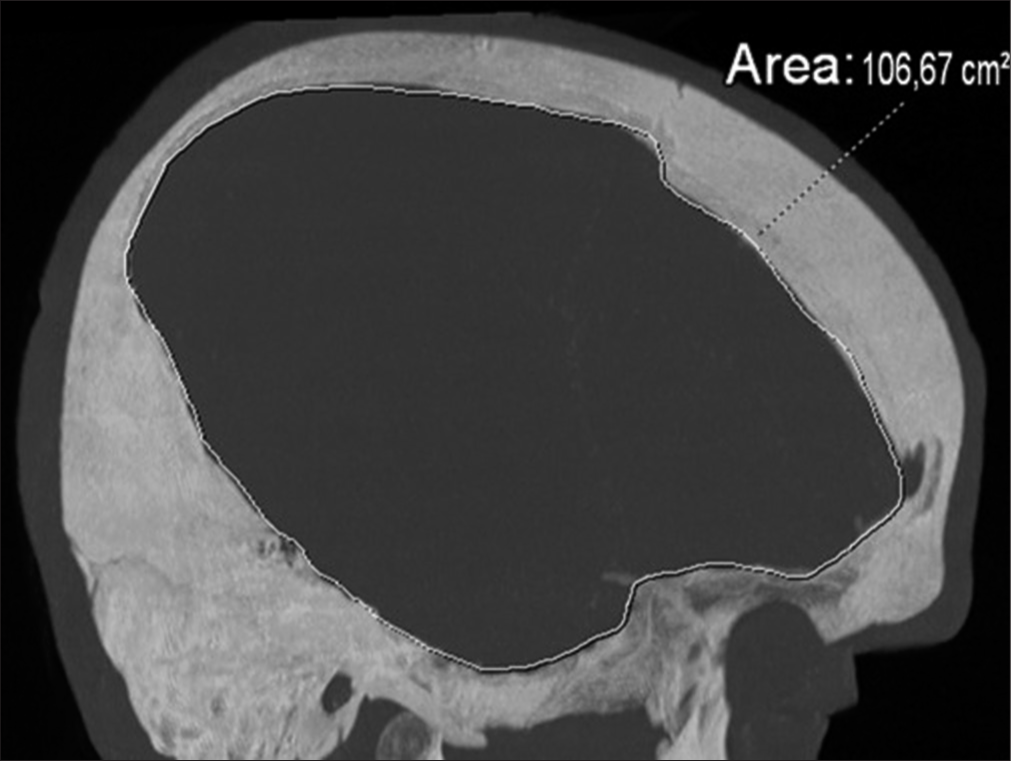
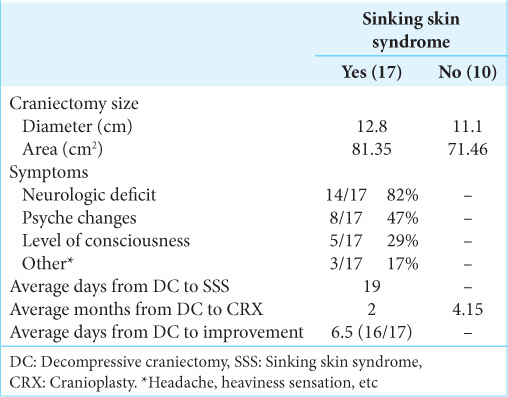
The diameter and area of the craniectomy were calculated. For the general population, the p50 diameter of the DC was 12.6 cm and the area of decompression was 78 cm2. In the group of patients with SSS, the p50 was 12.8 cm and for patients without SSS was 11.1 cm (z score = 0.32). We also calculated the area of decompression being 81.5 cm2 for the group of patients with SSS and 71.43 cm2 for the patients without the syndrome (z score = 0.61) [
Clinical features
Of our 27 patients, 17 (62.9%) presented SSS. The most common symptom for patients with SSS was neurologic deficit (82%) followed by psyche changes (47%) [
Radiologic features
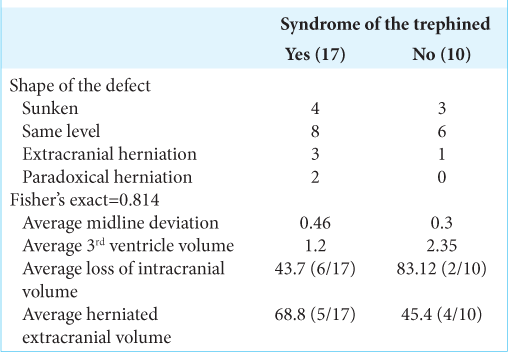
As described above, we classified our patients according to the shape of the craniectomy flap as «same level» (14 patients, 51.8%), «sunken» (seven patients, 25.9%), and «extracranial herniation» (four patients, 14.8%). Two patients (7.4%) presented the paradoxical herniation phenomena. Midline deviation was present in 12 (70.6%) patients with SSS and 7 (70%) without the syndrome [
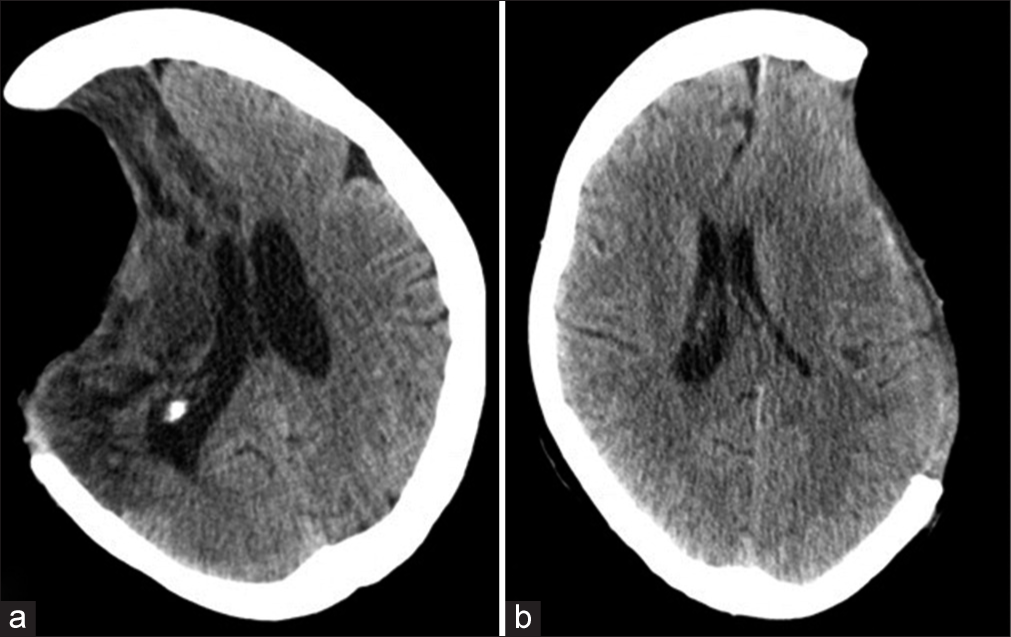
The sunken flap sign, defined as the observable skin depression at the craniectomy site, was present in six patients in the SSS group (35.2%) and one in the group without the syndrome (10%). The relative sinking volume was calculated in both groups, respectively, 43.76 versus 14.24 cc (z score= 0.5).
Extracranial herniation was observed in 5 (29.4%) patients with SSS and 2 (20%) in patients without the syndrome. The relative herniated volumes were calculated in both groups also: 68.8 versus 45.4 cc (z score = 0.8), respectively. The presence of paradoxical herniation was confirmed in two patients exclusively in the SSS group.
Finally, the third ventricle volume average in our series was 2.06 cc. In the group of patients with SSS this volume was 1.2 and in the group of patients without the syndrome it was 2.35 cc (z score = 0.04).
Time from DC to SSS
The average days from the craniectomy to the appearance of the syndrome were 19 days (p25 = 16 and p75 = 22) [
Time from DC to cranioplasty
The average days from the decompression to the cranioplasty were 3 months (p25 = 2 and p75 = 6.3) in the general population. According to our protocols, in our service, we try to replace the defect in the first 3–4 months following decompression whenever it is possible and there is no contraindication. In the group of patients with SSS, the time until cranioplasty was 4.1 months (p50) and for patients without the syndrome was 2 months (z score = 0.33) [
Improvement after cranioplasty
In our series, 94.11% of patients (16 out of 17) clearly improved after replacement of the cranial defect. For improvement, we considered disappearance and/or alleviation of symptoms. The mean time to improvement was 6.5 days (p25 = 4.5 and p75 = 8.5) after cranioplasty. One patient did not improve at all after the procedure [
DISCUSSION
In our series, there were no differences between patients with and without SSS when age and sex were considered. Some studies have suggested a slightly male predominance,[
In the literature, there are several findings about the increased mean times from DC to cranioplasty (usually ≥9 months) being a factor for higher risk of presenting SSS.[
The craniectomy size has been suggested as a factor to be considered. There are no correlations published in the literature[
The most common clinical finding was full or some grade of motor weakness (82%). Since motor involvement is one of the most easily recognized signs in this syndrome, some others can be underestimated. In that sense, most authors suggest that cognitive deficit should be actively reported. In our series, almost half of the patients with the SSS (47%) had some kind of cognitive involvement.
As the name suggests, the identification of a sunken flap is related to the presence of SSS. While many reports confirm this association,[
Radiological findings are not standardized and there is no radiology-based classification neither, but we can find wholesome descriptions about features of this syndrome. At present, we can only provide broad categories of radiological features based on the morphology of the defect as follows: (a) sunken, (b) same level, (c) extracranial herniation, and (d) paradoxical herniation and we propose that this nomenclature should be used while describing SSS. The use of a single terminology can help to report and describe this underestimated pathology.
Paradoxical herniation has been reported as a rare complication in these patients.[
CSF studies in patients after DC can explain some of the incidence of SSS. Besides the well-known variations in the systolic flow velocity due to cranioplasty, there is also the CSF volume loss that can aggravate or promote the appearance of SSS. In postcraniectomized patients, there is evidence that CSF leakage or over drainage can lead to neurological deterioration.[
Finally, the only treatment for this entity is the cranial defect replacement [
It is our practice to individualize patients and replace the cranial defect as early as we can considering all variables about diagnosis and probable complications.
CONCLUSION
The SSS is an underestimated and poorly understood entity not being actively reported nor investigated. While motor weakness is the main feature, cognitive deficit can also appear. Low 3rd ventricle volumes have a good relation with the presence of SSS, while measuring the relative cranial volumes (herniated and loss) could be useful in larger series to predict its development. The presence of a sunken flap does not guarantee SSS and is in the best of interest to establish some kind of classification based on radiological features: A = sunken, B = same level, C = extracranial herniation, and D = paradoxical. Although there is a hot debate about when to replace the cranial defect, we suggest it is vital to consider doing it early on after a DC.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akins PT, Guppy KH. Sinking skin flaps, paradoxical herniation, and external brain tamponade: A review of decompressive craniectomy management. Neurocrit Care. 2008. 9: 269-76
2. Ashayeri K, Jackson EM, Huang J, Brem H, Gordon CR. Syndrome of the trephined. A systematic review. Neurosurgery. 2016. 79: 525-34
3. Di Rienzo A, Colasanti R, Gladi M, Pompucci A, Della Costanza M, Paracino R. Sinking flap syndrome revisited: The who, when and why. Neurosurg Rev. 2020. 43: 323-35
4. Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: Cosmetic or therapeutic?. Surg Neurol. 1997. 47: 238-41
5. Fodstad H, Love JA, Ekstedt J, Fridén H, Liliequist B. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir (Wien). 1984. 70: 21-30
6. Gadde J. Syndrome of the trephined (sinking skin flap syndrome) with and without paradoxical herniation: A series of case reports and review. Del Med J. 2012. 84: 213-8
7. Gardner WJ. Closure of defects of the skull with tantalum. Surg Gynecol Obstet. 1945. 80: 303-12
8. Guido LJ, Patterson RH. Focal neurological deficits secondary to intraoperative CSF drainage: Successful resolution with an epidural blood patch report of two cases. J Neurosurg. 1976. 45: 348-51
9. Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939. 110: 488-512
10. Grantham EG, Landis HP. Cranioplasty and the post-traumatic syndrome. J Neurosurg. 1948. 5: 19-22
11. Honeybul S, Janzen C, Kruger K, Ho KM. The impact of cranioplasty on neuroological function. Br J Neurosurg. 2013. 27: 636-41
12. Jeyaraj P. Importance of early cranioplasty in reversing the syndrome of the trephine/motor trephine syndrome/sinking skin flap syndrome. J Maxillofac Oral Surg. 2015. 14: 666-73
13. Joseph V, Reilly P. Syndrome of the trephined. J Neurosurg. 2009. 111: 650-2
14. Kolias AG, Kirkpatrick PJ, Hutchinson PJ. Decompressive craniectomy: Past, present and future. Nat Rev Neurol. 2013. 9: 405-15
15. Langfitt TW. Increased intracranial pressure. Clin Neurosurg. 1969. 16: 436-71
16. Paredes I, Castaño-León AM, Munarriz PM, Martínez-Perez R, Cepeda S, Sanz R. Cranioplasty after decompressive craniectomy. A prospective series analyzing complications and clinical improvement. Neurocirugia (Astur). 2015. 26: 115-25
17. Sarov M, Guichard JP, Chibarro S, Guettard E, Godin O, Yelnik A. Sinking skin flap syndrome and paradoxical herniation after hemicraniectomy for malignant hemispheric infarction. Stroke. 2010. 41: 560-2
18. Sedney CL, Dillen W, Julien T. Clinical spectrum and radiographic features of the syndrome of the trephined. J Neurosci Rural Pract. 2015. 6: 438-41
19. Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery. 1994. 34: 729-31
20. Schmidt JH, Reyes BJ, Fischer R, Flaherty SK. Use of hinge craniotomy for cerebral decompression. Technical note. J Neurosurg. 2007. 107: 678-82
21. Suzuki N, Suzuki S, Iwabuchi T. Neurological improvement after cranioplasty. Analysis by dynamic CT scan. Acta Neurochir (Wien). 1993. 122: 49-53
22. Vasung L, Hamard M, Soto MC, Sommaruga S, Sveikata L, Leemann B. Radiological signs of the syndrome of the trephined. Neuroradiology. 2016. 58: 557-68
23. Yamaura A, Sato M, Meguro K, Nakamura T, Uemura K. Cranioplasty following decompressive craniectomy-analysis of 300 cases (author’s transl). No Shinkei Geka. 1977. 5: 345-53
24. Yoshida K, Furuse M, Izawa A, Iizima N, Kuchiwaki H, Inao S. Dynamics of cerebral blood flow and metabolism in patients with cranioplasty as evaluated by 133Xe CT and 31P magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry. 1996. 61: 166-71
25. Zhao J, Li G, Zhang Y, Zhu X, Hou K. Sinking skin flap syndrome and paradoxical herniation secondary to lumbar drainage. Clin Neurol Neurosurg. 2015. 133: 6-10