- Department of Neurosurgery, Odense University Hospital, Odense,
- Department of Neurosurgery, Rigshospitalet, Copenhagen, Denmark.
Correspondence Address:
Anders Blach Naamansen, Department of Neurosurgery, Odense University Hospital, Odense, Denmark.
DOI:10.25259/SNI_1119_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anders Blach Naamansen1, Carl Christian Larsen2, Bjarni Johannsson1, Sune Munthe1, Troels Halfeld Nielsen1. Small ruptured intracranial aneurysms are overrepresented at the anterior and posterior communicating artery: Results of a multiple regression analysis. 08-Jul-2022;13:288
How to cite this URL: Anders Blach Naamansen1, Carl Christian Larsen2, Bjarni Johannsson1, Sune Munthe1, Troels Halfeld Nielsen1. Small ruptured intracranial aneurysms are overrepresented at the anterior and posterior communicating artery: Results of a multiple regression analysis. 08-Jul-2022;13:288. Available from: https://surgicalneurologyint.com/surgicalint-articles/11710/
Abstract
Background: Anterior communicating artery (AcomA) represents the most common location for ruptured intracranial aneurysms (rIAs). Approximately 50% of all rIAs are smaller than 7 mm, but factors that lead to rupture are multifactorial. The study investigates whether AcomA location represents an independent risk factor for small size at time of rupture (
Methods: The aSAH cohort was retrospectively searched from our institution charts. The cohort was dichotomized into small aneurysms (
Results: One-hundred and seventy-six patients were included in the study. About 49.4% of the aneurysms were P = 0.006), internal carotid artery (other than PcomA) (P = 0.013), and posterior circulation (P = 0.017), when controlling for risk factors.
Conclusion: Ruptured AcomA and PcomA aneurysms are more frequent smaller than 7 mm compared to other locations. Patients with unruptured UIA at either AcomA or PcomA may be at increased risk of rupture even if the size of the aneurysm is small. Further studies are needed to confirm this finding.
Keywords: Rupture risk, Small aneurysms, Unruptured intracranial aneurysm
INTRODUCTION
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating event with a mortality of more than 30% and morbidity of around 70%.[
MATERIALS AND METHODS
The study was approved by our Institutional Review Board. According to local and national legislation, patient informed consent was not required for this study. All medical records of patients treated for SAH on our institution between November 2015 and December 2018 were retrospectively reviewed. Patients were identified using the ICD-10 codes I60.1–I60.9. All patients with SAH caused by other etiology than an intracranial aneurysm were excluded along with patients with arteriovenous malformation associated SAH or aneurysms. If the aneurysm causing SAH was not of the saccular type, the patients were excluded and patients with perioperative or postoperative aneurysm rupture were excluded as well. If the medical records lacked adequate information to assess variables included in the UIATS, patients were also excluded from the study. All variables from the original UIATS score were included in the present study except “Fear of rupture,” neurocognitive disease, psychiatric disorder, and life expectancy due to chronic or malignant disorder, as these variables either cannot be assessed retrospectively or does not increase the risk of rupture per se. The relevant medical records were reviewed by a single investigator (AN), scoring all patients according to the UIATS, using all definitions as described in Etminan et al.[
Statistical analysis
Age is presented as median and full range. Variables of the UIATS are presented as count and frequency. Size was dichotomized to <7.0 mm or larger than 7.0 mm. Superior cerebellar artery, basilar artery bifurcation, posterior cerebral artery, vertebral artery, and PICA were consolidated to a single variable labeled “posterior circulation.” Logistic univariate regression analysis was performed with size as the dependent variable and with patient and aneurysm characteristics as independent variables. Variables of interest presenting with P ≤ 0.2 along with patient characteristics were included in a logistic multiple regression. These were location, age (represented as a continuous variable in the analysis, divided by 10 such that odds ratios are fixed in increments of 10 years), gender, hypertension, current cigarette smoking, aneurysm multiplicity, and aneurysm morphology. The variable morphology was designed as a binary composite variable including irregularity or lobulation, size ratio >3, and/or aspect ratio >1.6. Only one of the criteria needed to be present. Aneurysm location was defined as a categorical variable represented by a level each in the model. Statistically significant results are presented as such with respect to an unadjusted α = 0.05 to protect against type-2 errors.
Data availability
All anonymized data will be shared by request from any qualified investigator.
RESULTS
One-hundred and seventy-six patients were included in the study. A flowchart of patient exclusion is given in
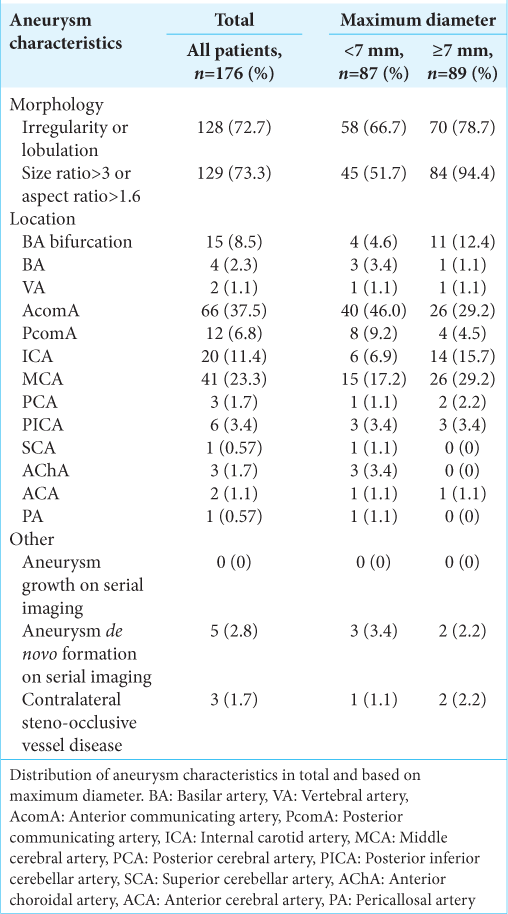
Patient characteristics
Patient demographics, characteristics, treatment modality, and clinical outcome are given in
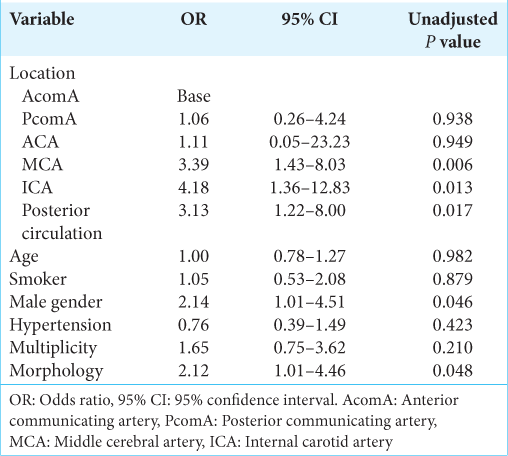
Aneurysm characteristics
For distribution of aneurysm characteristics see
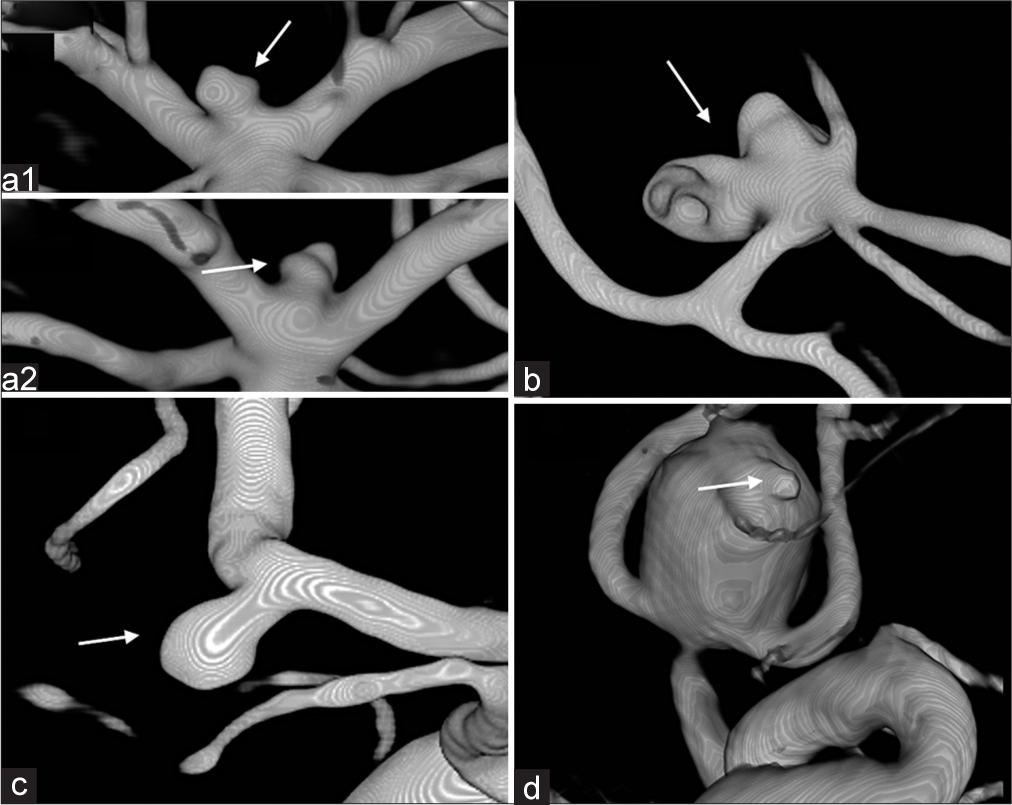
Multiple regression analysis
Aneurysm location, gender, and morphology were found to be significantly associated with ruptured aneurysm size in the multiple regression analysis [
DISCUSSION
A central dogma in the assessment of intracranial aneurysm rupture risk is that size matter. The larger the aneurysm, the higher the rupture risk. The ISUA study was the first to suggest a cutoff of 7 mm, below which the rupture risk is small. In fact, the study demonstrated a 0.05% 5-year rupture risk for aneurysms <7 mm in the anterior circulation.[
Limitations
The study has limitations. First, it is a retrospective study and the cohort subject to selection bias. Only patients who presented to hospital and underwent radiologic workup were included in the study. Accordingly, patients with aSAH who never presented to the hospital were not included in the study. It has been estimated that around 12% of aSAH patients die before they reach medical care with the majority of aneurysms located in the posterior circulation.[
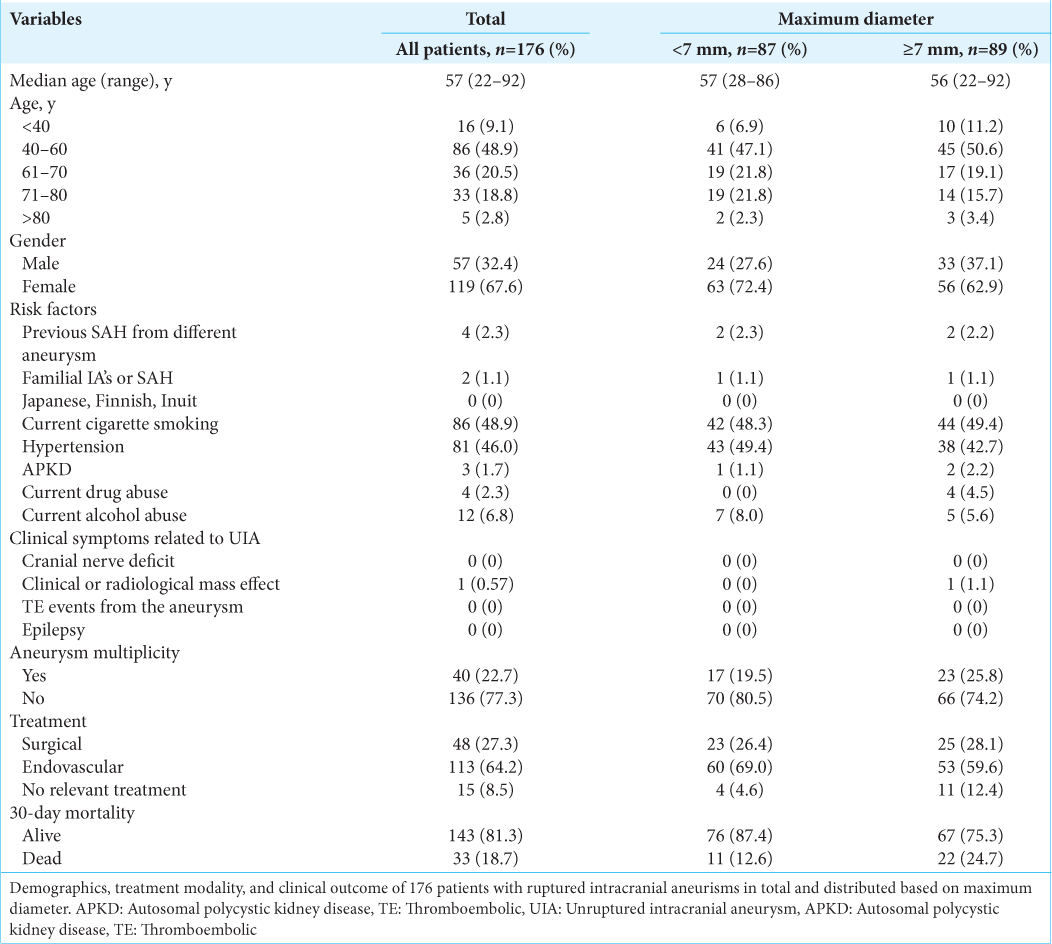
Figure 2:
Aneurysm morphology examples and in doubt cases. (a1+a2) Aneurysm of doubt regarding irregularity/lobulation (arrows). Scored as irregular/lobulated. (b) Aneurysm evaluated to have significant lobulation (arrow), giving it points for lobulation, but also for high complexity-related risk. (c) Aneurysm with no irregularity or lobulation (arrow). (d) Aneurysm scored to have lobulation. Based on the spherical shape apart from the assumed bleeding point (arrow), it might have been without lobulation prerupture.[
CONCLUSION
In the present study of an aSAH cohort, small aneurysms <7 mm are significantly more frequently located at the AcomA and PcomA than other locations when controlling for other known risk factors. This could be of use for clinicians when counseling patients regarding preventive treatment or conservative management, but further studies are needed to validate this finding.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bender MT, Wendt H, Monarch T, Beaty N, Lin LM, Huang J. Small aneurysms account for the majority and increasing percentage of aneurysmal subarachnoid hemorrhage: A 25-year, single institution study. Neurosurgery. 2018. 83: 692-9
2. Bijlenga P, Ebeling C, Jaegersberg M, Summers P, Rogers A, Waterworth A. Risk of rupture of small anterior communicating artery aneurysms is similar to posterior circulation aneurysms. Stroke. 2013. 44: 3018-26
3. Cebral J, Ollikainen E, Chung BJ, Mut F, Sippola V, Jahromi BR. Flow conditions in the intracranial aneurysm lumen are associated with inflammation and degenerative changes of the aneurysm wall. AJNR Am J Neuroradiol. 2017. 38: 119-26
4. Choi JH, Park HS. The incidence and characteristics of patients with small ruptured aneurysms (<5 mm) in subarachnoid hemorrhage. J Korean Neurosurg Soc. 2017. 60: 424-32
5. Etminan N, Brown RD, Beseoglu K, Juvela S, Raymond J, Morita A. The unruptured intracranial aneurysm treatment score: A multidisciplinary consensus. Neurology. 2015. 85: 881-9
6. Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MD, Rinkel GJ. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: A systematic review and meta-analysis. JAMA Neurol. 2019. 76: 588-97
7. Froelich JJ, Neilson S, Peters-Wilke J, Dubey A, Thani N, Erasmus A. Size and location of ruptured intracranial aneurysms: A 5-year clinical survey. World Neurosurg. 2016. 91: 260-5
8. Gasparotti R, Liserre R. Intracranial aneurysms. Eur Radiol. 2005. 15: 441-7
9. Greebe P, Rinkel GJ, Hop JW, Visser-Meily JM, Algra A. Functional outcome and quality of life 5 and 12.5 years after aneurysmal subarachnoid haemorrhage. J Neurol. 2010. 257: 2059-64
10. Greving JP, Wermer MJ, Brown RD, Morita A, Juvela S, Yonekura M. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014. 13: 59-66
11. Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke. 1997. 28: 660-4
12. Huang J, van Gelder JM. The probability of sudden death from rupture of intracranial aneurysms: A meta-analysis. Neurosurgery. 2002. 51: 1101-5
13. Inagawa T, Hirano A. Autopsy study of unruptured incidental intracranial aneurysms. Surg Neurol. 1990. 34: 361-5
14. 15. Mocco J, Brown RD, Torner JC, Capuano AW, Fargen KM, Raghavan ML. Aneurysm morphology and prediction of rupture: An international study of unruptured intracranial aneurysms analysis. Neurosurgery. 2018. 82: 491-6 16. Pagiola I, Mihalea C, Caroff J, Ikka L, Chalumeau V, Iacobucci M. The PHASES score: To treat or not to treat? Retrospective evaluation of the risk of rupture of intracranial aneurysms in patients with aneurysmal subarachnoid hemorrhage. J Neuroradiol. 2020. 47: 349-52 17. Rahman M, Ogilvy CS, Zipfel GJ, Derdeyn CP, Siddiqui AH, Bulsara KR. Unruptured cerebral aneurysms do not shrink when they rupture: Multicenter collaborative aneurysm study group. Neurosurgery. 2011. 68: 155-60 18. Rinaldo L, Nesvick CL, Rabinstein AA, Lanzino G. Differences in Size between unruptured and ruptured saccular intracranial aneurysms by location. World Neurosurg. 2020. 133: e828-34 19. Schneiders JJ, Marquering HA, van den Berg R, VanBavel E, Velthuis B, Rinkel GJ. Rupture-associated changes of cerebral aneurysm geometry: High-resolution 3D imaging before and after rupture. AJNR Am J Neuroradiol. 2014. 35: 1358-62 20. Skodvin TO, Johnsen LH, Gjertsen O, Isaksen JG, Sorteberg A. cerebral aneurysm morphology before and after rupture: Nationwide case series of 29 aneurysms. Stroke. 2017. 48: 880-6 21. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82 22. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36 23. Weir B, Disney L, Karrison T. Sizes of ruptured and unruptured aneurysms in relation to their sites and the ages of patients. J Neurosurg. 2002. 96: 64-70 24. Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: An updated meta-analysis. Stroke. 2007. 38: 1404-10 25. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 362: 103-10 26. Wiebers DO. Unruptured intracranial aneurysms: Natural history and clinical management. Update on the international study of unruptured intracranial aneurysms. Neuroimaging Clin N Am. 2006. 16: 383-90 27. Yi J, Zielinski D, Chen M. Cerebral aneurysm size before and after rupture: Case series and literature review. J Stroke Cerebrovasc Dis. 2016. 25: 1244-8 28. Zhang X, Karuna T, Yao ZQ, Duan CZ, Wang XM, Jiang ST. High wall shear stress beyond a certain range in the parent artery could predict the risk of anterior communicating artery aneurysm rupture at follow-up. J Neurosurg. 2018. 131: 868-75