- Department of Neurosurgery, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, IMSS, Ciudad de México, Mexico.
- Department of Anatomopathology, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, IMSS, Ciudad de México, Mexico.
Correspondence Address:
Eric Misael Estrada Estrada, Department of Neurosurgery, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, IMSS, Ciudad de México, Mexico.
DOI:10.25259/SNI_812_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Luis David Molina Andaluz1, Josué Alejandro Cervantes Gonzalez1, Zita Elizabeth Salazar Ramírez1, Nelly Ramírez2, Luis Guillermo Castellanos2, Eric Misael Estrada Estrada1. Solitary bone plasmacytoma as posterior fossa cranial neoplasia, presentation of two clinical cases. 05-Jan-2022;13:7
How to cite this URL: Luis David Molina Andaluz1, Josué Alejandro Cervantes Gonzalez1, Zita Elizabeth Salazar Ramírez1, Nelly Ramírez2, Luis Guillermo Castellanos2, Eric Misael Estrada Estrada1. Solitary bone plasmacytoma as posterior fossa cranial neoplasia, presentation of two clinical cases. 05-Jan-2022;13:7. Available from: https://surgicalneurologyint.com/surgicalint-articles/11330/
Abstract
Background: Solitary bone plasmacytoma is a plasmatic cell dyscrasia; its presentation in the posterior fossa is very rare.
Case Description: We present two cases, a 59-year-old male and a 50-year-old female, both with heterogeneous clinical presentation. One had symptoms compatible with endocranial hypertension, and the other presented with a hemispheric cerebellar syndrome and ipsilateral trigeminal neuralgia. They were both related to an intraosseous tumor of the occipital region near the torcula with large extension to the posterior fossa. The diagnosis of a plasma cell neoplasm arising from the diploe of the squamous portion of the occipital bone was confirmed with immunohistochemistry.
Conclusion: The treatment for a cranial tumor that is suspected to be a solitary bone plasmacytoma requires a multidisciplinary team to diagnose, plan a total resection, and after surgery continue with the follow-up of the patient. Solitary bone plasmacytoma should be considered as a differential diagnosis for a tumor that produces cancellous bone widening without sclerotic borders.
Keywords: Intraosseous cranial tumor, Plasmatic cell tumor, Posterior cranial fossa, Solitary bone plasmacytoma
INTRODUCTION
Plasmacytoma is a rare plasma cell neoplasm that represents <10% of monoclonal gammopathies.[
In the skull, the most affected regions are skull base, sphenoclival, petrous apex, nasopharynx, and orbital roof. It is less frequent in the calvaria (0.7% of the total)[
CASE PRESENTATION
Case 1
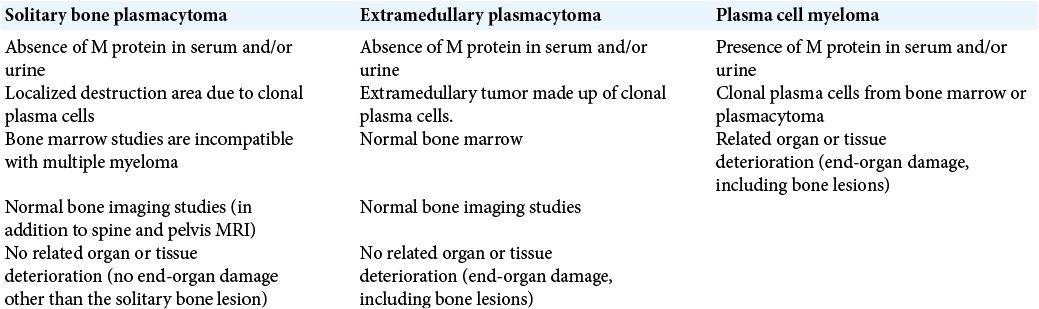
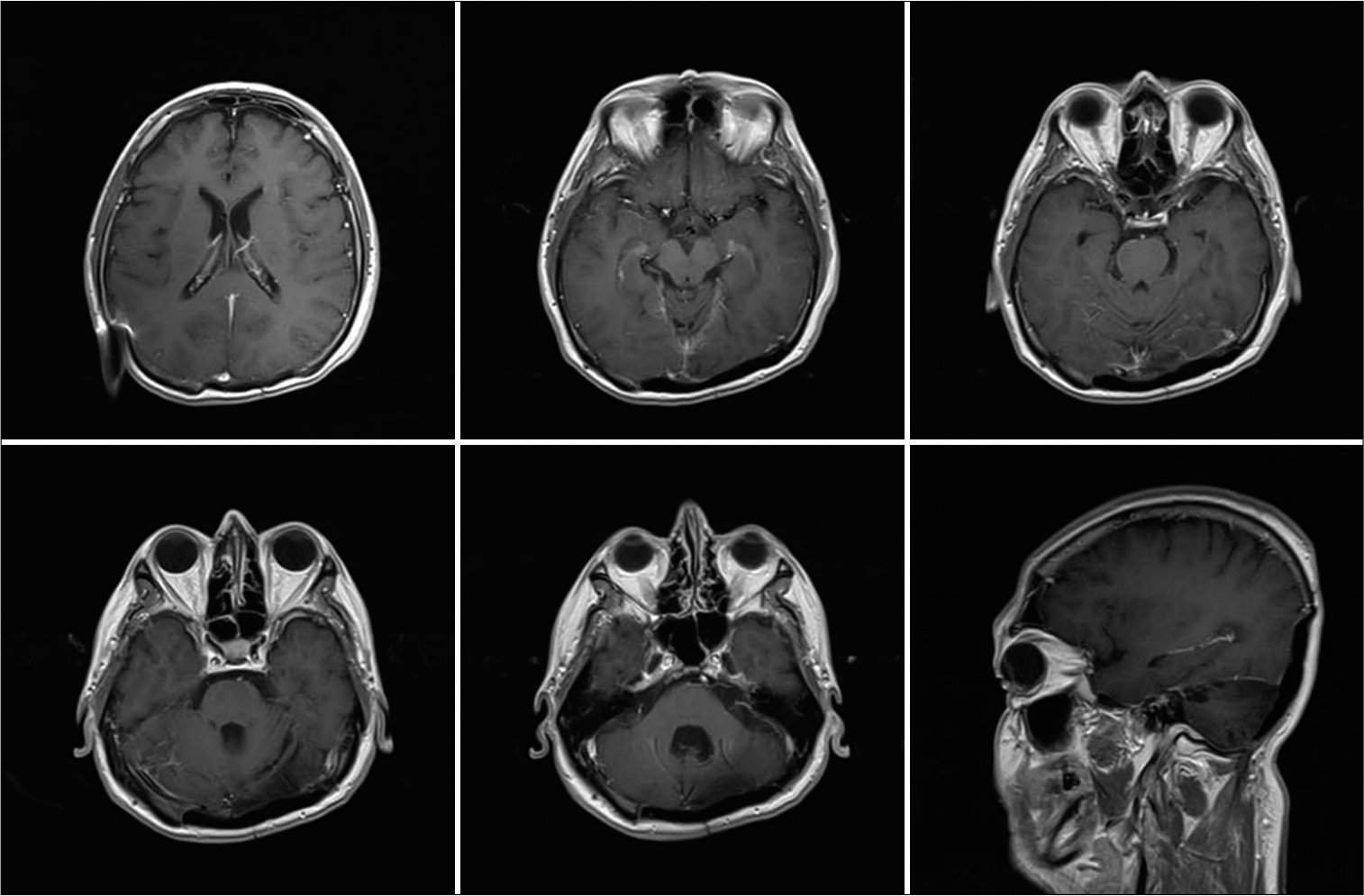
Case 1: Fifty-nine-year-old male, without relevant prior medical history, began in April 2018 with a frontal oppressive headache of moderate intensity; he was initially diagnosed with hypertension and received treatment with calcium channel blockers. He was initially diagnosed with hypertension and received treatment with calcium channel blockers. In August 2018, he started noticing unsteady gait, disorientation, and intermittent urinary incontinence. He sought medical attention at his regional hospital in December 2018, where an unenhanced CT brain scan was diagnostic for obstructive hydrocephalus secondary to a posterior fossa tumor. A right precoronal ventriculoperitoneal high-pressure shunt was placed, apparently without complications. He was sent for consultation to our unit in February 2019. At his initial physical examination, a right hemispheric cerebellar syndrome was documented. In the initial MRI, a posterior fossa tumor was observed, of approximately 61 × 60 × 52 mm, with irregular borders, isointense in T1- and T2-weighted images, with homogenous enhancement on contrast-enhanced images; with compression and displacement of the fourth ventricle and the cerebellar left hemisphere obliteration of the right transverse sinus was also documented [
Figure 1:
T1-weighted contrast-enhanced brain MRI, where skull invasion to the posterior fossa, the squamous portion of the occipital bone and both parietal bones can be appreciated. The fourth ventricle is displaced to the right and partially obliterated; there is also significant compression of the cerebellar left hemisphere, as well as obliteration of the right transverse sinus on the magnetic resonance angiography.
In June 2019, surgery was performed. A combined supra- and infra-tentorial approach with a bilateral parieto-occipital craniectomy, ligation of the right transverse sinus, total resection of the tumor, duraplasty, and cranioplasty with polymethylmethacrylate was performed. A solid, extra-axial tumor of a rubbery consistency, with invasion to parietal bone, occipital squama in its three layers, right transverse sinus, torcular Herophili, tentorium, and adjacent dura, was found.
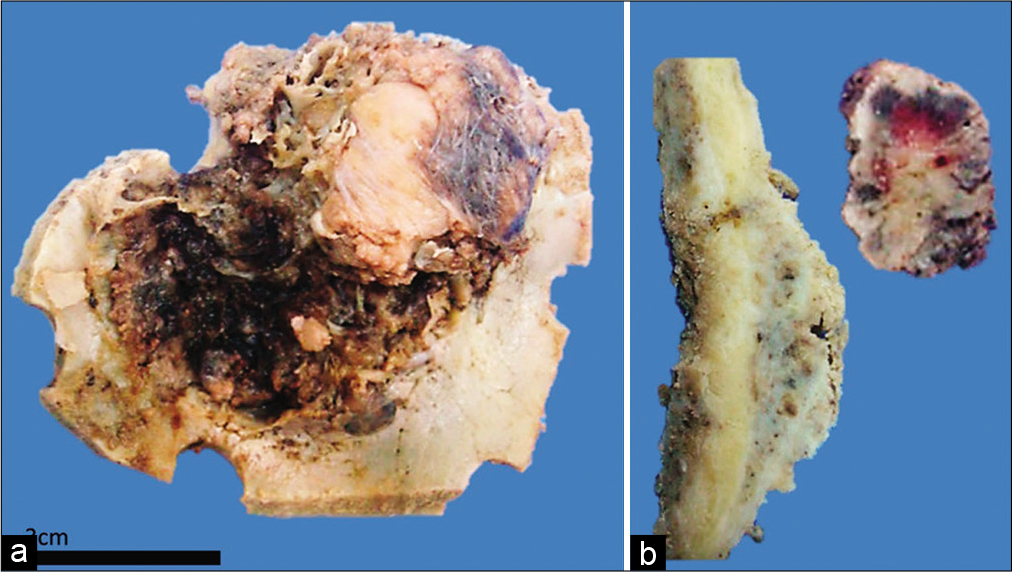
At the pathology laboratory, bone fragments with an approximate diameter of 8 cm were processed. A tumor which expanded the diploe up to several centimeters, with an external smooth surface and an internal fractious surface was observed. When cut, a separation created by the tumor between the internal and the external periosteum was made evident [
Figure 2:
Macroscopic image of Case 1 where osseous involvement can be appreciated. (a) Internal side of a fragment of occipital bone. A lesion with irregular borders and recent hemorrhage can be observed. It is infiltrative and fixed to the inner table. (b) The sample has been cut perpendicular to the lesion. One can recognize the infiltration and deformation to the external table, there is also a fragment of tumor which is not fixed to the bone with hemorrhaging areas.
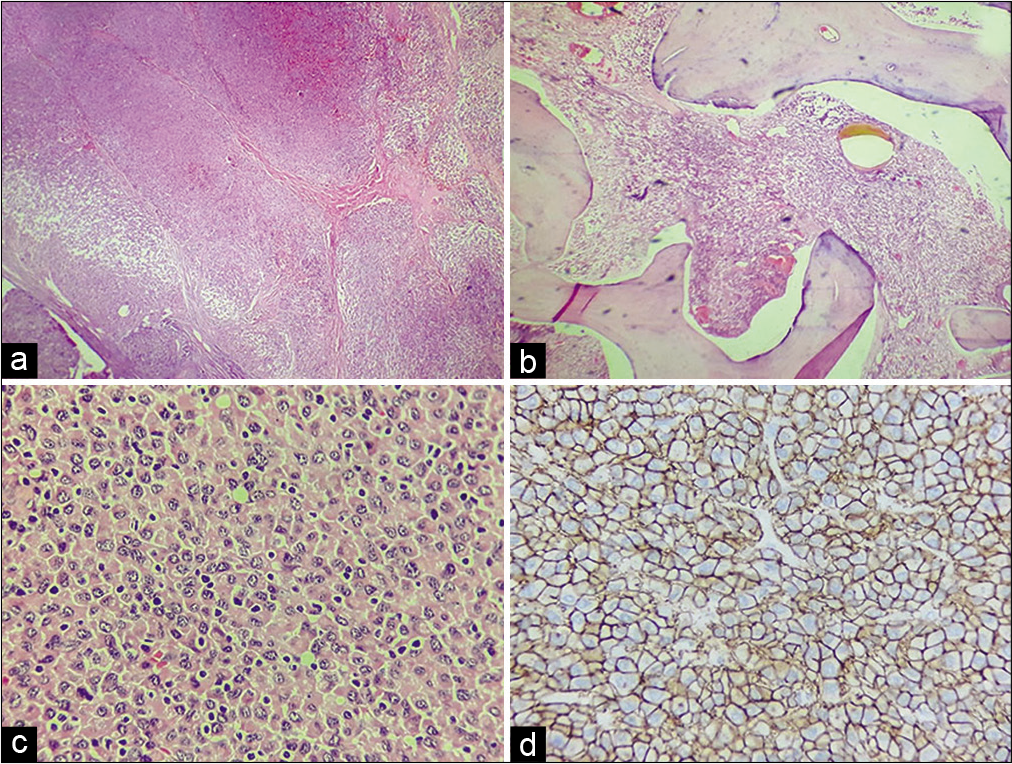
Figure 3:
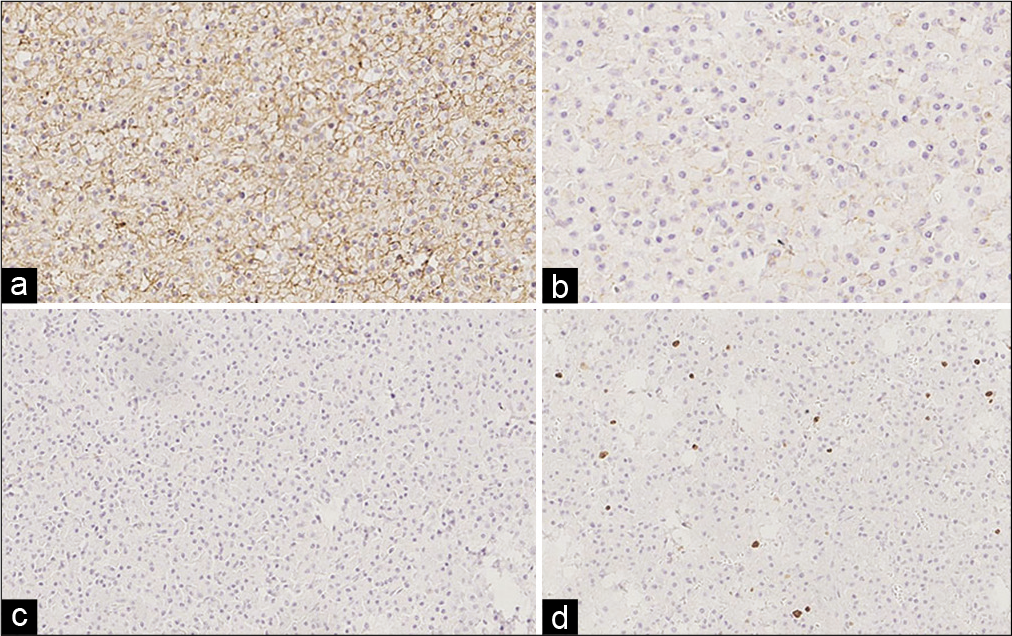
Photomicrograph of Case 1. (a) Panoramic image which shows sheets of neoplastic cells separated by fibrous septa (HE; ×50), (b) they infiltrate the medullary cavity of trabecular bone (HE; ×100). (c) Close-up which shows plasmacytoid cells and atypical plasma cells associated to small, reactive lymphocytes (HE, ×200). (d) IHC for CD138 which is positive in the cytoplasmic membrane (immunoperoxidase, ×250).
The patient showed a satisfactory postoperative evolution and was discharged a week after. Afterward, treatment was continued with 28 fractionated craniospinal radiotherapy sessions in December 2019. At his 6-month follow-up consultation, he was found to be disease free [
Case 2
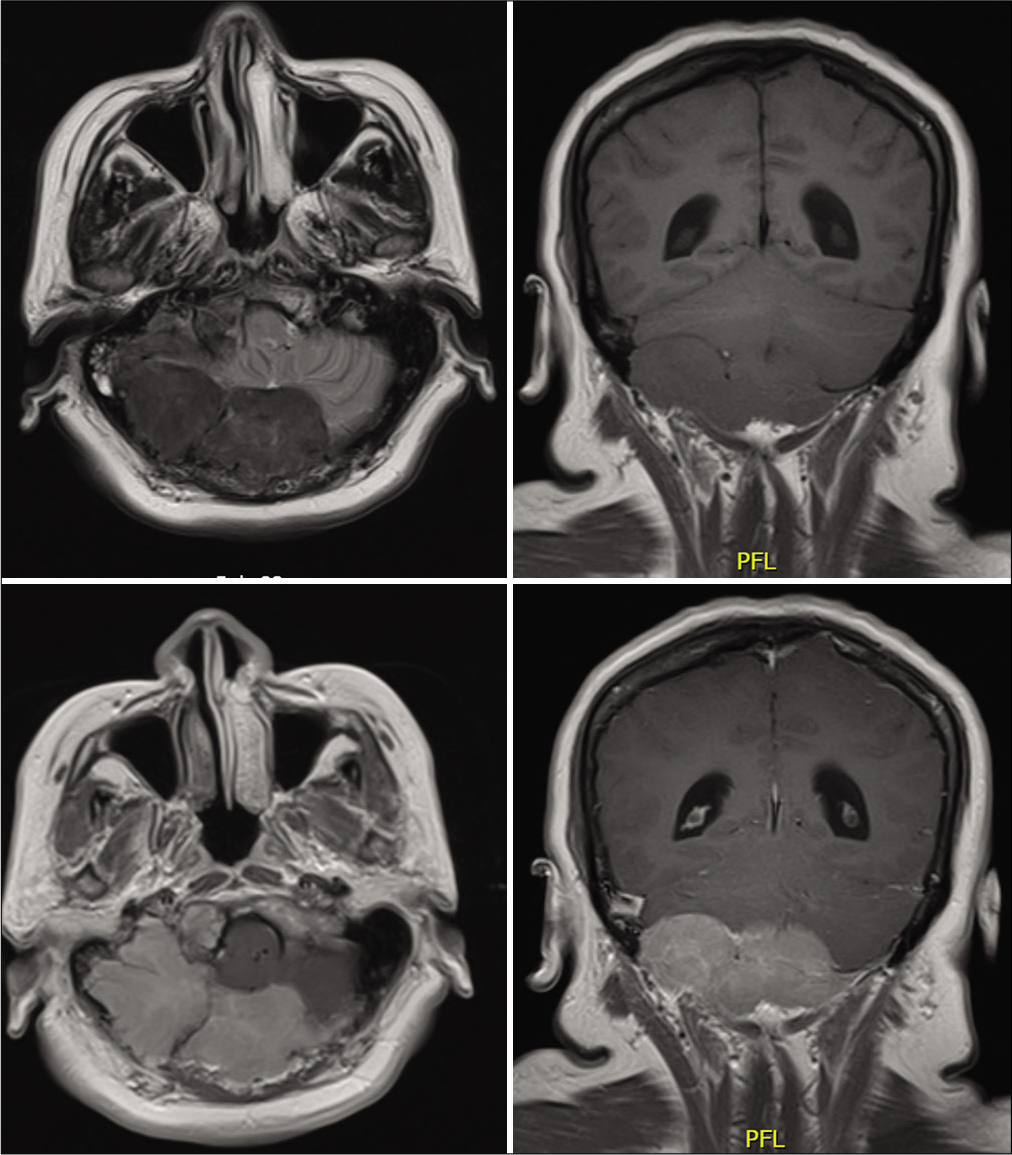
A 50-year-old female with prior medical history of smoking and controlled hypertension, presented to clinic with an 18-month history of a moderate intensity, occipital, and oppressive headache, it partially improved with NSAIDs. The headache was accompanied with gait lateropulsion, right peripheral facial palsy, and right hemifacial electric shock-like pain, as well as paresthesia. On initial head MRI, there was a posterior fossa tumor, which extended from the right petroclival fissure to the occipital squama, of approximately 88 × 51 × 40 mm, with irregular borders, isointense in T1-weighted images and hypointense in T2-weighted images, with homogenous enhancement on contrast-enhanced images, it caused descending compression of the cerebellar right hemisphere and encased the exiting roots of cranial nerves V, VII-VIII. [
In January 2018, a suboccipital craniectomy and subtotal resection of the tumor were performed. A soft, extradural, neovascularized tumor, which eroded the adjacent occipital squama, was found. The patient had no postsurgical complications.
At the pathology laboratory, irregular, soft, fractious, brownish-white tissue fragments were processed; they measured 6 × 5 × 1.5 cm approximately. No bone fragments were processed. The histopathological analysis showed a neoplasm which was made up of plasma cells; most were mature, with minimal variation in nuclear morphology. IHC: CD138-positive diffuse expression 3+/3+; CD79-positive focal expression 2+/3+; CD19-positive focal expression 2+/3+ (<5%); CD56-positive diffuse expression 2+/3+; CD117 and cyclin D1 negative; kappa negative; lambda-positive focal expression 3+/3+ (5%); and proliferative rate (Ki-67) 5% [
DISCUSSION
SBP presenting as a posterior fossa tumor is extremely rare,[
Approximately 5% of patients with multiple myeloma (MM) are initially diagnosed with SBP.[
The clinical-radiological differential diagnosis for a tumor in the posterior fossa includes meningiomas, sarcomas, primary bone tumors, and metastatic carcinoma,[
Due to its common location in the skull base, these tumors frequently affect the cranial nerves. Other reported clinical presentations are headache and intracranial mass effect.[
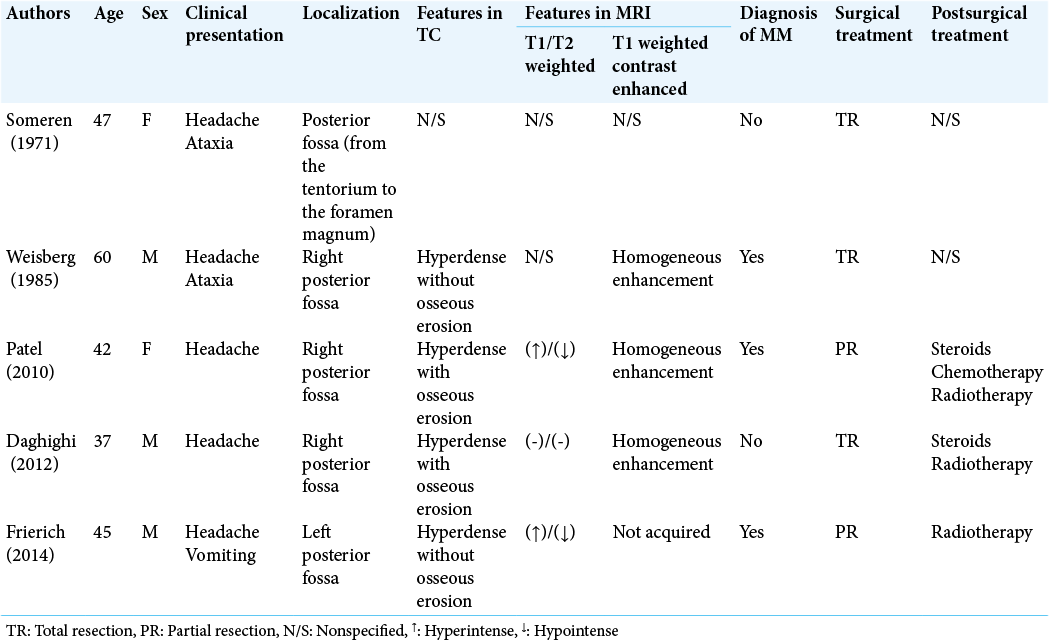
Regarding radiological presentation, plasmacytomas present in an unenhanced CT scan as osteolytic lesions without a sclerotic periphery. SBP presents on MRI as an enhancing lesion, iso- or hyperintense on T1-weighted images, and iso- or hypointense in T2-weighted images. These characteristics were observed in our patients’ CTs and MRIs. On an unenhanced CT scan, the lesions’ sharp edges, lack of sclerosis, and minimal periosteal reaction strongly correlate with a plasmacytoma. This suggests that a paramount study to identify bone remodeling is unenhanced CT scan [
Figure 7:
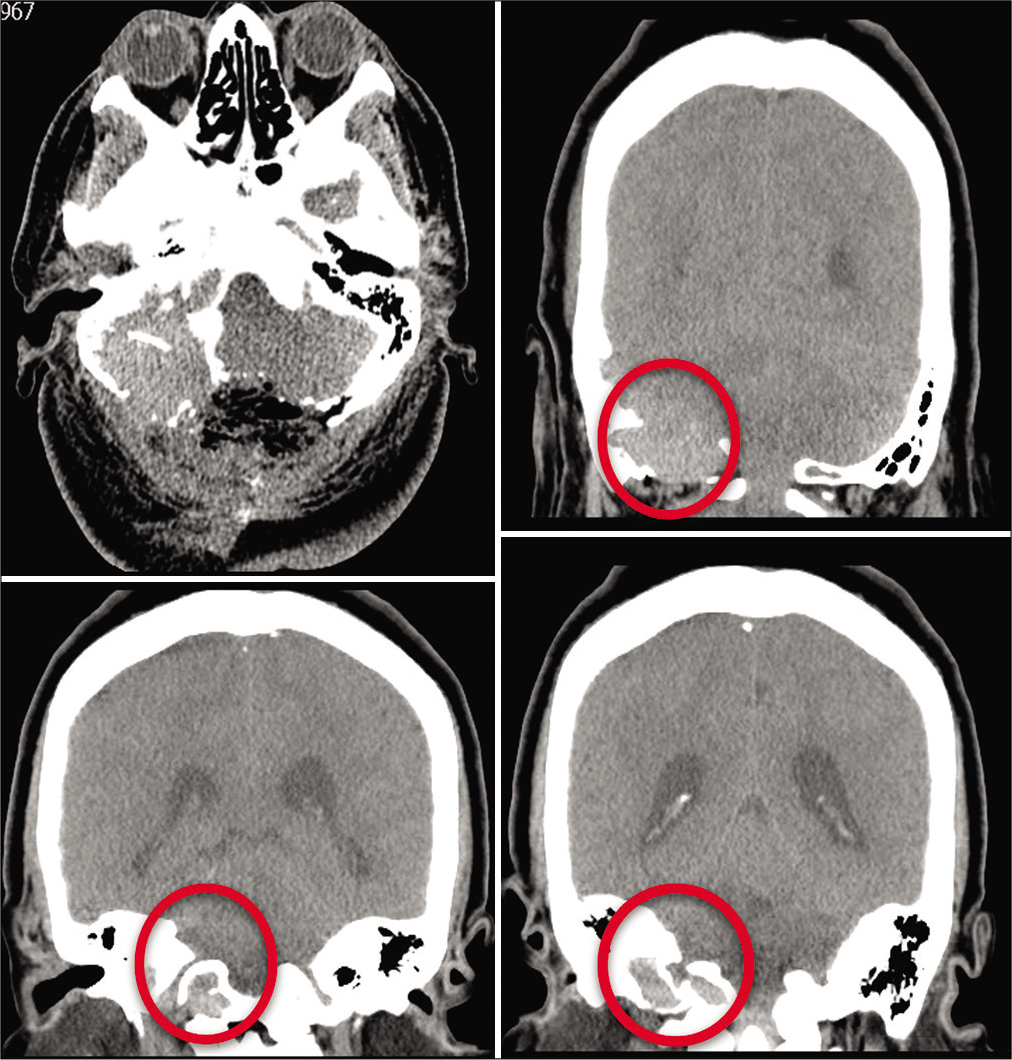
Six-month postoperative CT scan of Case 2 in axial and coronal views. Bilateral progressive widening of the cancellous bone of the squamous portion of the occipital bone and of the left condyle can be observed. There is also invasion to the adjacent petrous bone. It has been referred to as an osteolytic lesion without a sclerotic border. There is residual unresectable tumor due to blood loss during surgery.
In the histopathological study, plasmacytoma is a malignant proliferation of sheets of plasma cells with few scattered cells of different lineage. Tumors can be made up of small mature plasma cells with small round nuclei, their chromatin grouped peripherally; but they can also have bigger cells with a plasmablastic morphology, with big, atypical nuclei, vesicular chromatin patters, and prominent central nucleoli. Cytological atypia is variable. The cytoplasm is moderately abundant in most cases, eccentric, and may contain inclusions of condensed immunoglobulins.[
After an SBP diagnosis, patients need to be evaluated to rule out MM with serum electrophoresis, Bence-Jones protein in urine, PET scan, and bone marrow biopsy;[
After a bibliographical review of surgical resection of plasmacytomas, we found in an article from Na’ara et al. that a R0 resection (microscopically margin-negative tumor bed) was achieved in 45% of the patients, R1 resection (microscopic margins positive for tumor) in 18% and R2 resection (gross residual tumor) in 37%.[
There is evidence that if a R0 resection is achieved, radiotherapy can be withheld until there is tumor recurrence. Radiotherapy generally requires a 40–50 Gy dose administered over 20–25 fractions over a 4-week period. This is adjusted depending on the tumor’s size. Favorable response to radiotherapy is usually over 80%.[
A progression to MM from SBP has been established, thus chemotherapy should be initiated in patients in which this progression is documented.[
CONCLUSION
Treatment for a cranial tumor that is suspected to be SBP requires a multidisciplinary team to diagnose, plan a total resection and after surgery continues with the follow-up of the patient. SBP should be considered as a differential diagnosis for a tumor that produces osseous changes, which can be particularly observed in a unenhanced CT brain scan [
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alappatt JP, Anto D, Ajayakumar A. Falcotentorial plasmacytoma: A case report. Surg Neurol. 2004. 62: 178-9
2. Bouali S, Taallah M, Bahri K, Zehani A, Abderrahmen K, Kallel J. Solitary plasmacytoma of the skull vault: A rare case report. Neurochirurgie. 2020. 66: 66-9
3. Daghighi MH, Poureisa M, Shimia M, Mazaheri-Khamene R, Daghighi S. Extramedullary plasmacytoma presenting as a solitary mass in the intracranial posterior fossa. Iran J Radiol. 2012. 9: 223-6
4. 5. Kumar S, Kimlinger T, Morice W. Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol. 2010. 23: 433-51 6. Ma XJ, Li D, Wang L, Hao SY, Zhang LW, Zhang JT. Clinical features, radiological profiles, and surgical outcomes of primary intracranial solitary plasmacytomas: A report of 17 cases and a pooled analysis of individual patient data. J Neurooncol. 2019. 142: 263-72 7. Munjal S, Srivastava A, Jain S, Mehta V. Skull vault plasmacytoma mimicking parasagittal meningioma: Mini-brain appearance. Asian J Neurosurg. 2019. 14: 231-3 8. Na’ara S, Amit M, Gil Z, Billan S. Plasmacytoma of the skull base: A meta-analysis. J Neurol Surg B Skull Base. 2016. 77: 61-5 9. Webb M, Barrett C, Barrett S, van Rensburg JJ, Louw V. Cranial plasmacytoma: A case series and review of the literature. Indian J Hematol Blood Transfus. 2013. 29: 43-7