- Department of Neurosurgery, Riuniti Hospital, Foggia, Italy,
- Division of Neurosurgery, Giovanni XXIII Hospital, Bari, Italy,
- Department of Neurosurgery, University of Foggia, Foggia, Italy,
- Department of Neurosurgery, Städtisches Klinikum Karlsruhe, Karlsruhe, Germany,
- Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy.
- Department of Radiological, Oncological and Pathological Sciences, Sapienza University of Rome, Roma, Italy.
- Department of Anatomical, Histological, Forensic Medicine and Orthopedic Sciences, Sapienza University of Rome, Roma, Italy.
Correspondence Address:
Augusto Leone, Department of Neurosurgery, Städtisches Klinikum Karlsruhe, Karlsruhe, Germany.
DOI:10.25259/SNI_297_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Antonio Colamaria1, Maria Blagia2, Matteo Sacco1, Savino Iodice1, Francesco Carbone3, Nicola Pio Fochi1, Augusto Leone4, Matteo Landriscina5, Giulia Coppola6, Elena De Santis7, Guido Giordano5. Solitary metastasis from renal cell carcinoma to the choroid plexus: A case illustration and review of the literature. 27-May-2022;13:227
How to cite this URL: Antonio Colamaria1, Maria Blagia2, Matteo Sacco1, Savino Iodice1, Francesco Carbone3, Nicola Pio Fochi1, Augusto Leone4, Matteo Landriscina5, Giulia Coppola6, Elena De Santis7, Guido Giordano5. Solitary metastasis from renal cell carcinoma to the choroid plexus: A case illustration and review of the literature. 27-May-2022;13:227. Available from: https://surgicalneurologyint.com/surgicalint-articles/11615/
Abstract
Background: Metastatic renal cell carcinoma (RCC) of the choroid plexus is an exceedingly rare condition, with only 35 reported cases to date. Surgical resection of these tumors poses a unique challenge to neurosurgeons since evidence-based treatment guidelines are yet to be designed.
Case Description: The authors describe the case of a 58-year-old woman presenting with progressive neurological deterioration 5 years after a right nephrectomy for a WHO 2016 Stage I RCC. A head, contrast-enhanced, and magnetic resonance revealed signs of obstructive hydrocephalus and a homogeneously contrast-enhancing 5 cm mass located in the trigone of the right lateral ventricle. Furthermore, a search of the literature was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. After screening for duplicates, 35 publications met the eligibility criteria. Finally, 17 manuscripts were included for analysis. Moreover, a detailed description of an illustrative case is provided. The median age at diagnosis for intraventricular metastasis from RCC was 62.9 years, showing a slight female prevalence. The lateral ventricles were reported as the most frequent location with only one patient presenting with obstructive hydrocephalus caused by the obliteration of Monro foramen. Management options included either open craniotomy or radiosurgery.
Conclusion: The management of choroid plexus metastasis from RCC is still controversial with various authors proposing different treatment strategies. In this article, in addition to an in-depth case description, a qualitative review of the literature on metastatic RCCs of the choroid plexus using the PRISMA is provided.
Keywords: Brain metastasis, Cerebrospinal fluid, Choroid plexus, Hydrocephalus, Renal cell carcinoma, Stereotactic radiosurgery
INTRODUCTION
Solitary metastases to the choroid plexus are very rare. Three large postmortem series have reported the frequency as being 0.9%, 2.6%, and 4.6% in patients with systemic cancer.[
In the present report, the authors describe the case of a metastatic RCC to the choroid plexus of the right lateral ventricle in a 58-year-old patient treated with total nephrectomy 5 years before presentation. In addition, the first systematic review of the available literature on RCC causing metastasis to the choroid plexus is provided.
METHODS
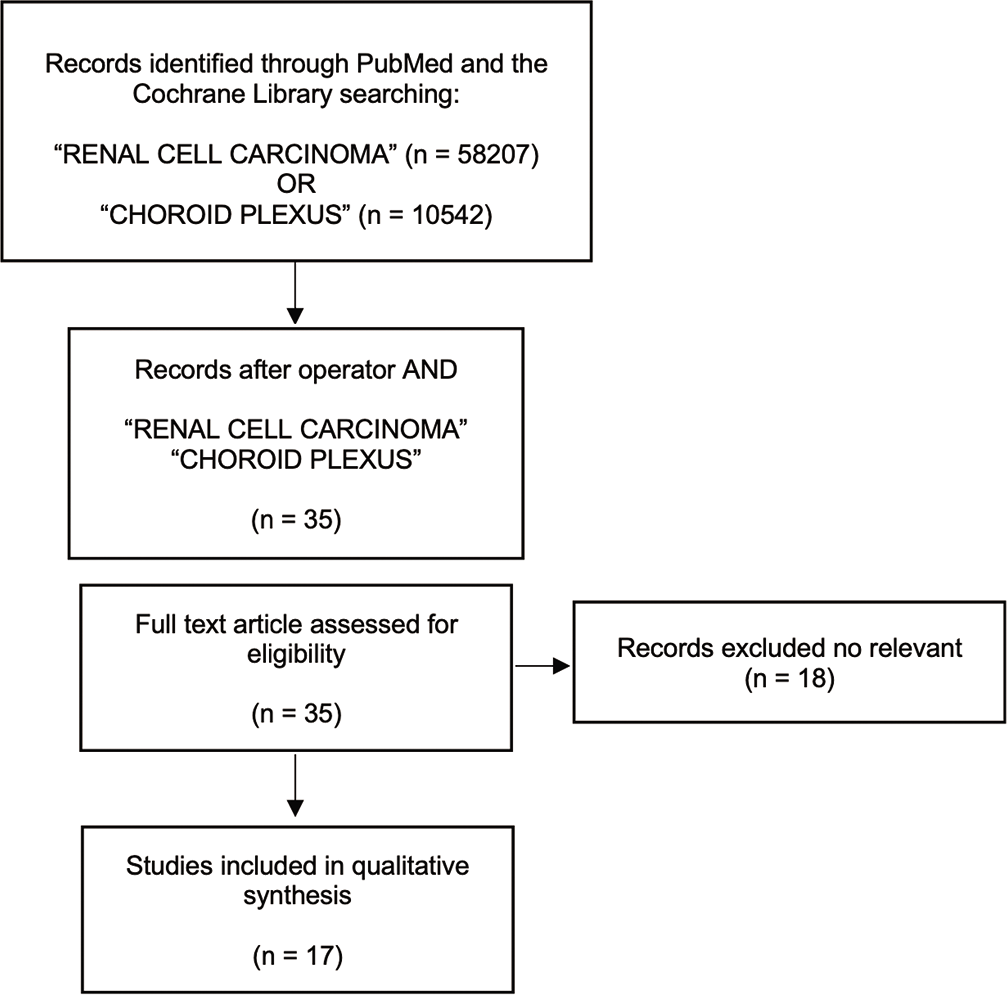
Patient’s medical history including comorbidities, concomitant medications, diagnosis, and treatment was taken from clinical records. Written informed consent for the case publication was obtained from the patient. A review of the literature was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Screening was performed by reviewing article titles or full text up to October 2021 using the electronic databases PubMed and the Cochrane Library. The primary search included “RCC” and “Choroid plexus” in the article titles. Only publications in the English language were included and the extracted citations were then screened for duplicates. Later operator “and” was applied on the extracted records by the use of the above-mentioned terms to narrow the scope of the review. Thirty-five articles met eligibility criteria for our qualitative systematic review and 18 were excluded because not relevant. Finally, 17 papers were included in the qualitative analysis [
ILLUSTRATIVE CASE
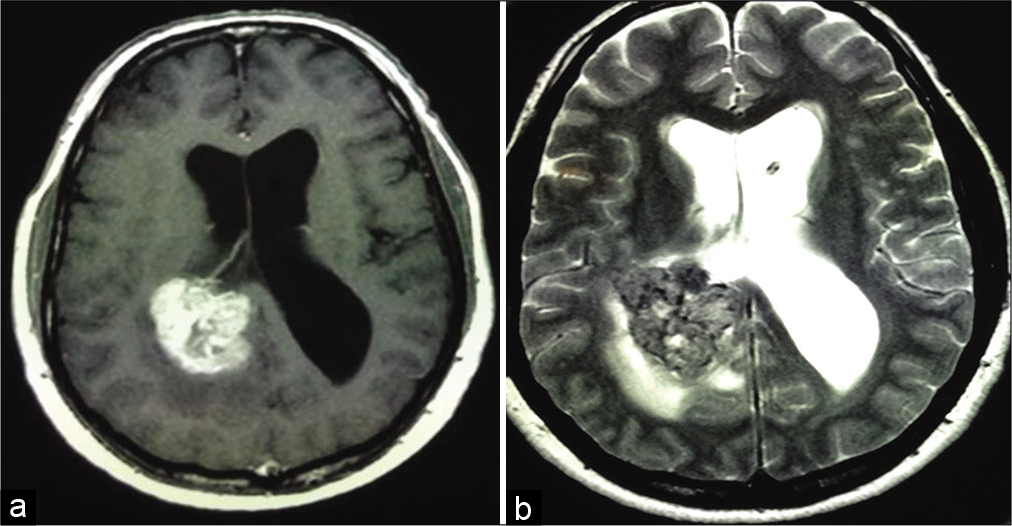
A 58-year-old right-handed woman presented to our attention with worsening headaches limiting her ability to perform daily tasks, gradual memory deficits, and progressive gait instability. The medical history was significant for a diagnosis of Stage I American Joint Committee on Cancer tumor-node-metastasis RCC that was treated with a right nephrectomy alone 5 years before presentation. At admission, her neurological examination was intact with no focal deficits and normal anal and urinary sphincter continence. A head, contrast-enhanced, and magnetic resonance imaging (MRI) investigation demonstrated a homogeneously contrast enhancing, 5 cm oval mass presumably originated from the choroid plexus in the trigone of the right lateral ventricle with signs of obstruction and dilation of the temporal horn and atrium of the contralateral ventricle [
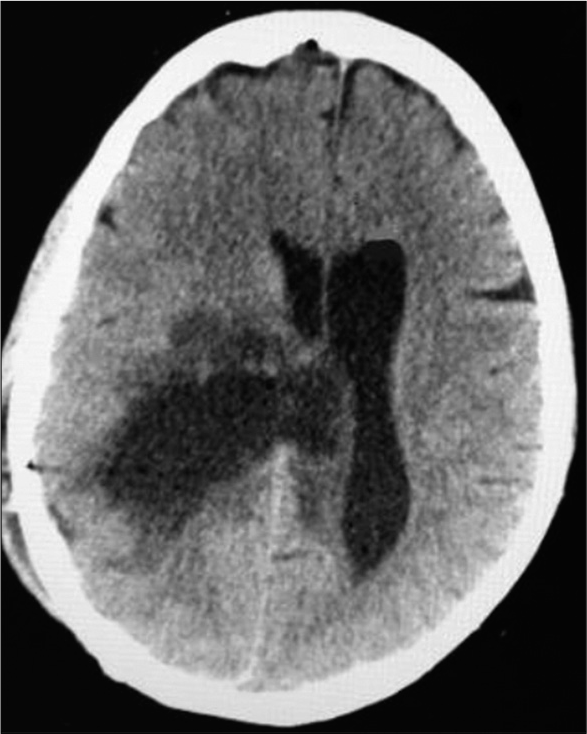
The postoperative period was unremarkable except for a transient left arm paresis that quickly subsided. The patient’s amnesia gradually improved, and the headaches disappeared, and she was discharged after a control computed tomography (CT) [
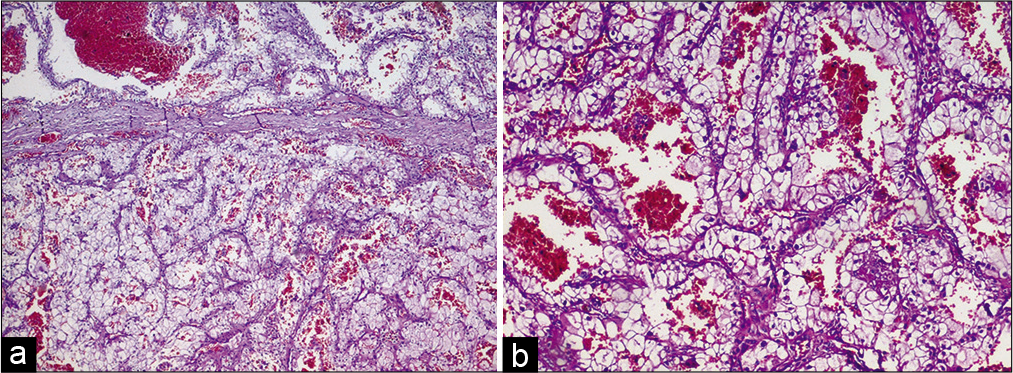
The final pathological examination revealed the peculiar pattern of gland tissue with nests of clear cells set within a sinusoidal vascular network [
DISCUSSION
Analysis of previously reported cases
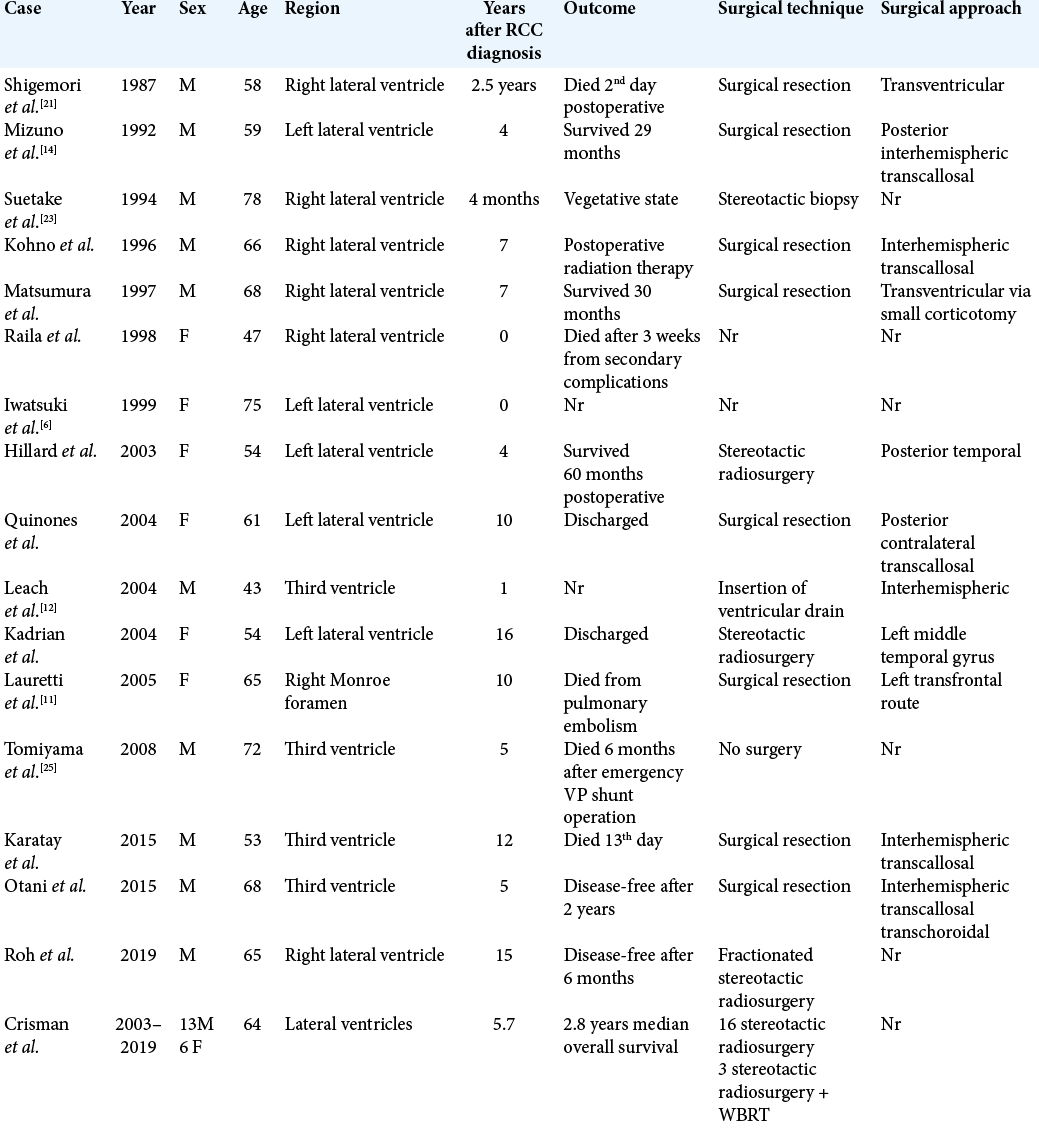
To date, 35 cases of solitary metastasis from RCC to the choroid plexus have been reported as summarized in [
The mean follow-up time of this population was 25.8 months (range: 2 weeks–30 months), with one postoperative death and one vegetative state. Survival times were not reported in 7 cases (23%), whereas in the cohort described by Crisman et al.,[
Epidemiology of RCC metastases to the ventricular system
Approximately 10–40% of patients diagnosed with cancer will eventually develop brain metastases over the course of their disease with 0.9–4.5% of malignancies found in the ventricular system.[
Metastasis to the choroid plexus
In line with the present review, Toms et al.[
Radiological and pathological diagnosis
For intraventricular metastases, the CT and MRI findings are nondefining, despite being crucial when planning for excision surgery or stereotactic radiosurgery (SRS), allowing the identification of the location, and the number of lesions. In general, solitary metastases from RCC to the choroid plexus appear hyperdense on CT scans and homogeneously contrast enhanced, surrounded by excessive vasogenic edema and intraventricular hemorrhage.[
Microscopic examination of choroid plexus metastases from RCC demonstrates nests of clear cells with the usual acinar pattern, along with hyperchromatic and pleomorphic nuclei contained in a clear cytoplasm and a stroma rich in small blood vessels.[
Current treatment and future perspectives
At present, there is no consensus over the treatment of intraventricular metastases and the preferred surgical approach is decided on several empirical factors including the location of the lesion, the size and number of tumors, and the patient’s performance status.[
CONCLUSION
Metastatic RCC of the choroid plexus represents an extremely rare entity. For instance, due to the lack of evidence-based guidelines, treatment options remain solely grounded on empirical data. This systematic review of the literature shows that, although not always feasible, SRS is generally more effective in terms of overall survival, tumor control rate, and postoperative recovery when compared with open surgical techniques.
Authors’ contributions’
AC, MS, ML, SI, and MB collected the case. NPF, FC, AL, and GG performed the literature research, wrote the paper, and assessed figures and tables. EDS reviewed and confirmed the histological diagnosis. GG, EDS, FC, AL, and NPF made the revision and supervised the project.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on reasonable request.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chakrabarti K, Swartz LK, Gill A, Fang F, Kidwell KM, Morikawa A. Development of CNS metastases in breast cancer patients treated with curative intent: A case-control study. CNS Oncol. 2020. 9: CNS61
2. Cheng Z, Chao Q, Zhang H, Wang DW, Shu HS. Intraventricular cystic papillary meningioma: A case report and literature review. Medicine (Baltimore). 2020. 99: e21514
3. Crisman CM, Patel AR, Winston G, Brennan CW, Tabar V, Moss NS. Clinical outcomes in patients with renal cell carcinoma metastases to the choroid plexus. World Neurosurg. 2020. 140: e7-13
4. Gutzmer R, Vordermark D, Hassel JC, Krex D, Wendl C, Schadendorf D. Melanoma brain metastases interdisciplinary management recommendations 2020. Cancer Treat Rev. 2020. 89: 102083
5. Hillard VH, Musunuru K, Hasan I, Zia S, Hirschfeld A. Long-term management of bilateral metastases of renal cell carcinoma to the choroid plexus. Acta Neurochir (Wien). 2003. 145: 793-7
6. Iwatsuki K, Sato M, Taguchi J, Fukui T, Kiyohara H, Yoshimine T. Choroid plexus metastasis of renal cell carcinoma causing intraventricular hemorrhage: A case report. No Shinkei Geka. 1999. 27: 359-63
7. Kadrian D, Tan L. Single choroid plexus metastasis 16 years after nephrectomy for renal cell carcinoma: Case report and review of the literature. J Clin Neurosci. 2004. 11: 88-91
8. Karatay M, Koktekir E, Erdem Y, Celik H, Bayar MA. Solitary metastasis of renal cell carcinoma to the third ventricle mimicking a colloid cyst: Case report. Turk Neurosurg. 2015. 25: 801-3
9. Klos KJ, O’Neill BP. Brain metastases. Neurologist. 2004. 10: 31-46
10. Kohno M, Matsutani M, Sasaki T, Takakura K. Solitary metastasis to the choroid plexus of the lateral ventricle. Report of three cases and a review of the literature. J Neurooncol. 1996. 27: 47-52
11. Lauretti L, Fernandez E, Pallini R, Massimi L, Albanese A, Denaro L. Long survival in an untreated solitary choroid plexus metastasis from renal cell carcinoma: Case report and review of the literature. J Neurooncol. 2005. 71: 157-60
12. Leach JC, Garrott H, King JA, Kaye AH. Solitary metastasis to the choroid plexus of the third ventricle mimicking a colloid cyst: A report of two cases. J Clin Neurosci. 2004. 11: 521-3
13. Matsumura H, Yoshimine T, Yamamoto S, Maruno M, Hayakawa T, Ono Y. Single solitary metastasis of the slowly progressive type of renal cell carcinoma to the choroid plexus--case report. Neurol Med Chir (Tokyo). 1997. 37: 916-9
14. Mizuno M, Asakura K, Nakajima S, Sampei T, Sayama I, Kawamura S. Renal cell carcinoma metastasizing to choroid plexus of lateral ventricle; A case report. No Shinkei Geka. 1992. 20: 469-74
15. Otani N, Wada K, Kumagai K, Takeuchi S, Nagatani K, Tomura S. Surgical removal of the solitary metastasis of renal cell carcinoma in the third ventricle using an interhemispheric transcallosal trans-choroidal approach. Asian J Neurosurg. 2015. 10: 58
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
17. Quinones-Hinojosa A, Chang EF, Khan SA, Lawton MT, McDermott MW. Renal cell carcinoma metastatic to the choroid mimicking intraventricular meningioma. Can J Neurol Sci. 2004. 31: 115-20
18. Raila FA, Bottoms WT, Fratkin JD. Solitary choroid plexus metastasis from a renal cell carcinoma. South Med J. 1998. 91: 1159-62
19. Roh H, Kim J, Chong K, Yoon WK, Kwon TH, Kim JH. Unexpected daily changes in tumor volume during fractionated gamma knife radiosurgery for solitary intraventricular metastatic renal cell carcinoma: A case report. Stereotact Funct Neurosurg. 2019. 97: 44-8
20. Shapira Y, Hadelsberg UP, Kanner AA, Ram Z, Roth J. The ventricular system and choroid plexus as a primary site for renal cell carcinoma metastasis. Acta Neurochir (Wien). 2014. 156: 1469-74
21. Shigemori M, Shimamoto H, Noguchi S, Yoshitake Y, Sugita Y, Kuramoto S. Choroid plexus metastasis of renal-cell carcinoma. Prog Comput Tomogr. 1987. 9: 603-6
22. Siomin V, Lin JL, Marko NF, Barnett GH, Toms SA, Chao ST. Stereotactic radiosurgical treatment of brain metastases to the choroid plexus. Int J Radiat Oncol Biol Phys. 2011. 80: 1134-42
23. Suetake K, Shinya T, Takeda M. A choroid plexus metastasis of a renal cell carcinoma: A case report. Jpn J Neurosurg (Tokyo). 1994. 3: 436-41
24. Thomas NJ, Myall NJ, Sun F, Patil T, Mushtaq R, Yu C. Brain metastases in EGFR-and ALK-positive NSCLC: Outcomes of central nervous system-penetrant tyrosine kinase inhibitors alone versus in combination with radiation. J Thorac Oncol. 2022. 17: 116-29
25. Tomiyama A, Nakayama H, Aoki K, Ueda M. Solitary metastasis of renal cell carcinoma to the third ventricular choroid plexus with rapid clinical manifestation by intratumoral hemorrhage. Neurol India. 2008. 56: 179-81
26. Toms SA, Suh JH, Weil RJ. Choroid plexus metastasis. Urology. 2007. 70: 370-1
27. Vecil GG, Lang FF. Surgical resection of metastatic intraventricular tumors. Neurosurg Clin N Am. 2003. 14: 593-606