- Division of Neurosurgery, Department of Neurology, University of Sao Paulo Medical School, Sao Paulo, Brazil.
Correspondence Address:
Vinicius Trindade Gomes da Silva, Department of Neurology, University of Sao Paulo, Sao Paulo, Brazil.
DOI:10.25259/SNI_165_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alexandra Gomes dos Santos, Wellingson Silva Paiva, Leila Maria da Roz, Marcelo Prudente do Espirito Santo, Manoel Jacobsen Teixeira, Eberval G. Figueiredo, Vinicius Trindade Gomes da Silva. Spheno-orbital meningiomas: Is orbit reconstruction mandatory? Long-term outcomes and exophthalmos improvement. 22-Jul-2022;13:318
How to cite this URL: Alexandra Gomes dos Santos, Wellingson Silva Paiva, Leila Maria da Roz, Marcelo Prudente do Espirito Santo, Manoel Jacobsen Teixeira, Eberval G. Figueiredo, Vinicius Trindade Gomes da Silva. Spheno-orbital meningiomas: Is orbit reconstruction mandatory? Long-term outcomes and exophthalmos improvement. 22-Jul-2022;13:318. Available from: https://surgicalneurologyint.com/surgicalint-articles/11732/
Abstract
Background: Meningiomas correspond to one-third of all primary central nervous system tumors. Approximately 9% of them are spheno-orbital meningiomas (SOMs), presenting significant clinical symptoms as visual impairment and orbital esthetics. This article aims to evaluate exophthalmos’ improvement in a surgical series without orbital reconstruction.
Methods: We consecutively included all patients diagnosed with SOM, admitted to a single institution for 10 years. Surgical resection was the standard of care, associated or not with adjuvant radiation therapy. The radiological investigation included preoperative and postoperative head CT or MRI. We quantified proptosis through imaging.
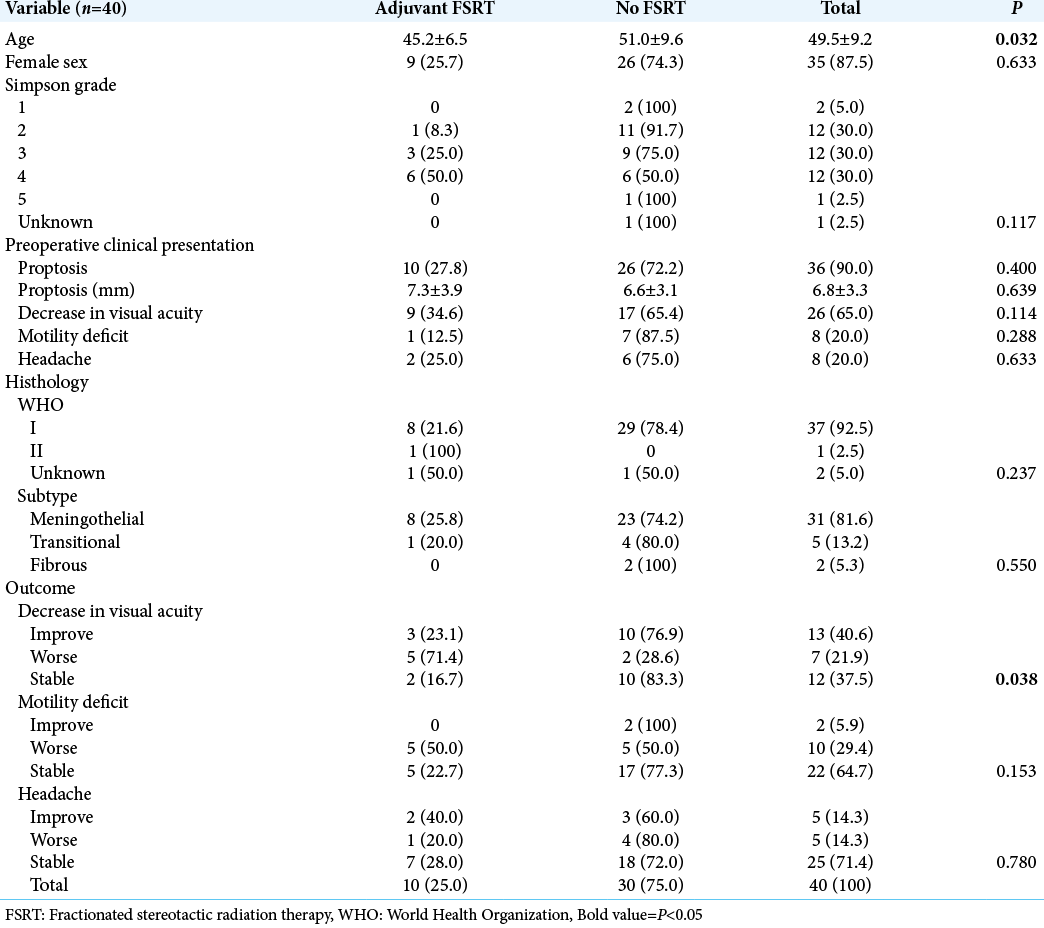
Results: Forty patients composed this series, 87.5% were female. Proptosis was the most common presentation (90%), followed by decreased visual acuity (65%), motility deficit (20%), and headache (20%). Gross total resection was achieved in 65% of the procedures. In late outcomes, 78% of the patients maintained or improved visual acuity and 85% maintained or improved headache. Proptosis significantly improved after surgery and along with the follow-up (P P = 0.038).
Conclusion: Resection of SOM was sufficient to stop the evolution of visual deficit and allowed the improvement of proptosis. Orbital reconstruction does not seem to be an essential step in reducing enophthalmos.
Keywords: Central nervous system tumor, Exophthalmos, Gross total resection, Meningioma, Orbit reconstruction, Spheno-orbital meningiomas
INTRODUCTION
Meningiomas correspond to one-third of all primary central nervous system (CNS) tumors. Approximately 9% of them are spheno-orbital meningiomas (SOMs).[
SOM management is challenging, since tumor grows into the cavernous sinus, with compression of the cranial nerves II, III, IV, V, and VI. The most related symptoms are decreased visual acuity, trigeminal neuralgia, diplopia, ptosis, and proptosis[
This article aims to evaluate exophthalmos’ improvement in a surgical series without orbital reconstruction and complications analysis. We discuss the preoperative presentation, surgical strategy, adjuvant treatment, and long-term outcomes.
MATERIALS AND METHODS
Setting
This retrospective case series consecutively included all patients older than 18 years, diagnosed with SOM who underwent surgical resection in a Brazilian single institution between 2008 and 2018. The exclusion criteria were patients who lost the follow-up. Informed consent for use of the radiological images obtained from each patient at the time of surgery, in accordance with the ethical guidelines approved by our university (research protocol 200/05). The patients consented to the procedures. Medical records provided us with demographic information, tumor characteristics, treatment details, and tumor progression, which were recorded under the research protocol. A total of 40 patients were included and 11 patients (27.5%) underwent the first resection in other services and were reoperated in this series.
Surgical management
We retrospectively reviewed perioperative clinical data records from a large brain tumor databank. The radiological investigation included pre- and postoperative head CT and MRI of the skull and orbit.
The standard of care was a pretemporal craniotomy, associated with superolateral orbitotomy for tumor resection [
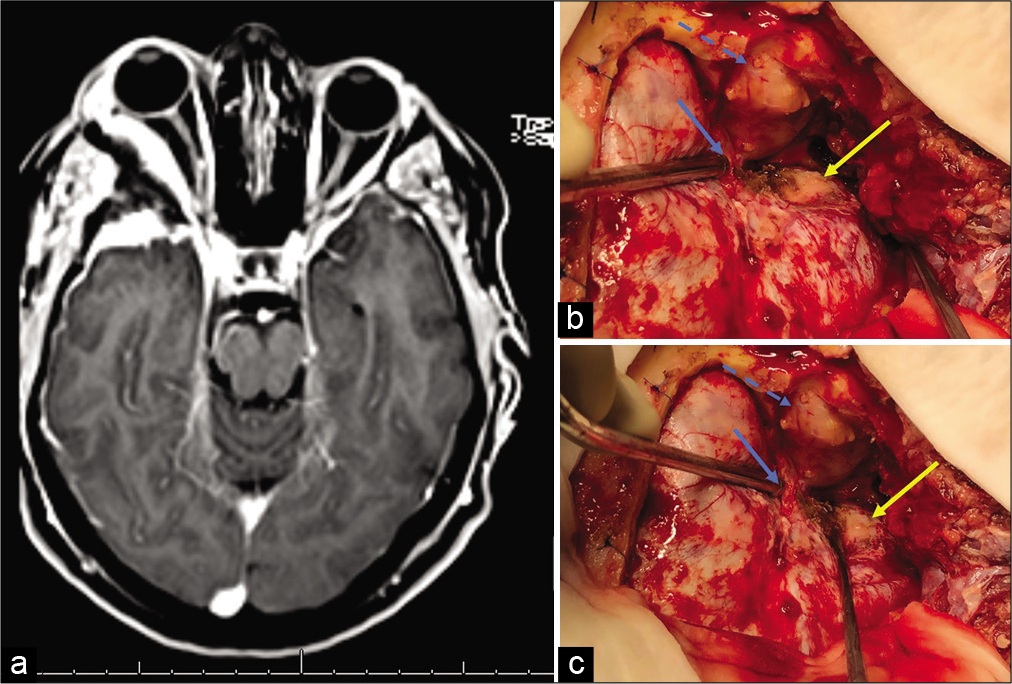
Figure 1:
(a) MRI-T1 gadolinium: right spheno-orbital meningioma. (b and c) Standard surgical strategy. We performed a pretemporal craniotomy. Blue arrow: superior orbital fissure exposed; blue dashed arrow: periorbita visualization after orbitotomy and intraorbital tumor resection; yellow arrow: tumor implantation. The greater sphenoid wing was drilled and removed.
In our service, we do not perform reconstruction of orbital walls after orbital decompression. The association of adjuvant fractionated stereotactic radiotherapy (FSRT) was made under the attending team’s discretion, considering the following factors: age, recurrence, and postoperative visual acuity. The FSRT protocol applied 50 Gy to the tumor bed in 28 sessions with 1.8 Gy/fraction.
Histopathological analysis categorized tumors into five different subtypes (meningothelial, transitional, fibrous, fibroblastic, and microcystic), graded according to the WHO Classification of Tumors of the CNS at the time of diagnosis.[
Outcomes
The primary outcome analyzed was proptosis improvement after the surgery. Proptosis assessment is essential in orbital reconstruction in craniofacial anomalies. We use an axial head computed tomography with a thin slice. Preoperative proptosis was measured using the exophthalmos index (EI) as presented by Scarone et al.[
We selected axial images in the orbital plane of the CT scan and MRI on the optic nerve level bilaterally. A single examiner analyzed all CTs/MRIs, placing a transverse tangent on the anterior lateral orbital rim of both sides and calculating the distance from the corneal center to the tangent line at a 90° angle. Then, we compared the difference in length (mm) between the pre- and postoperative scans to evaluate the exophthalmos’ improvement [
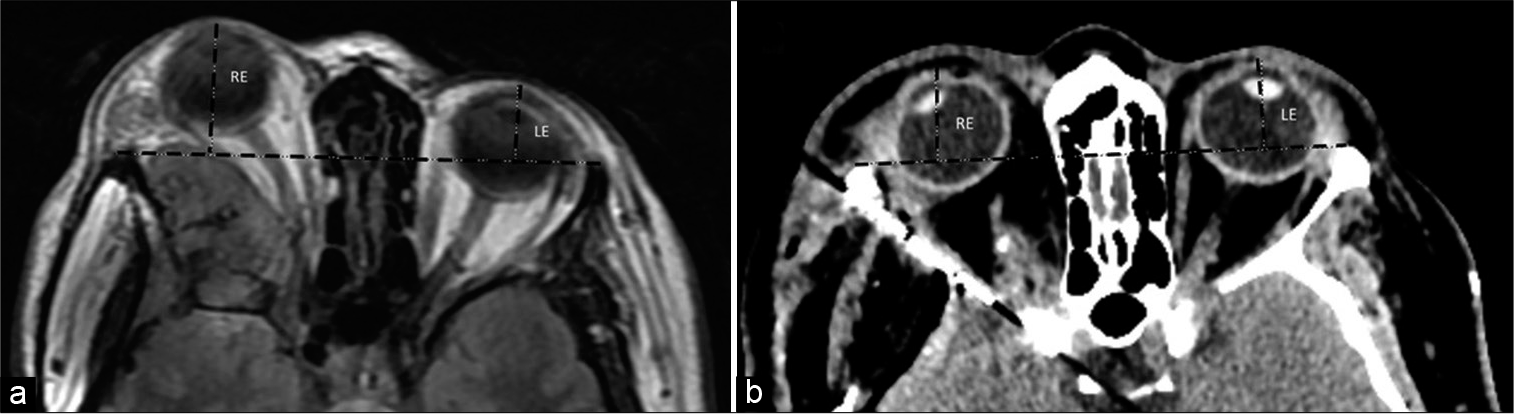
Figure 2:
A 53 y/o patient presenting right exophthalmos and decreased visual acuity. (a) Preoperative MRI showing a spheno-orbital meningioma, with a quantifiable proptosis of 10.8 mm. (b) Early CT scan after a Simpson II resection, with the absence of deviation. RE: right eye, LE: left eye. Black dashed lines representing exophthalmos index as presented by Scarone et al.
As secondary outcomes, we verified visual acuity, ocular motility, and campimetry exclusively through physical examination. All the 40 patients were examined by a neurosurgeon and an ophthalmologist. In addition, the incidence of headache and perioperative complications was recorded.
Statistical analysis
We described categorical variables with absolute and relative frequencies. Continuous variables were described with mean and standard deviations (for variables with normal distribution) and median with interquartile range (for nonnormal data). We assessed normal distribution through the methods of asymmetry and kurtosis. To compare the baseline characteristics of the stratified subgroups by recurrence and adjuvant radiotherapy, the Fisher’s exact test was used for categorical variables and the Mann– Whitney U-test for independent samples or for continuous variables as applicable. We analyzed variation of proptosis along the study using ANOVA for repeated measures. The significance level was <0.05. All analyses were performed using the software SPSS (IBM SPSS Statistics for Windows, version 24.0. Armonk, NY: IBM Corp.).
RESULTS
A total of 40 patients composed this case series; 87.5% were female. The mean age at surgery was 49.5 ± 9.2 years. All patients presented symptoms related to the tumor at the preoperative clinical evaluation. Twenty-nine patients had newly diagnosed meningiomas, while 11 patients presented recurrences of previously resected tumors. Proptosis was the most common presentation (90%), followed by decreased visual acuity (65%), motility deficit (20%), and headache [20%, Table 1]. Six patients had severe vision loss, two with only luminous perception, and four patients with amaurosis. A decrease of the visual campus was present in three patients with temporal hemianopsia. Finally, two patients presented seizures.
Regarding surgical resection, GTR was achieved in 65% of the procedures. We used periosteum grafts for dural reconstruction. Thirty-nine cases were diagnosed with the WHO Grade I meningiomas and only one patient with the WHO Grade II (atypical) meningioma. The most prevalent histology subtype was the meningothelial (81%). Perioperative complications included three surgical site infections, three postoperative seizures, two strokes, one pulmonary thromboembolism, and one patient with insipidus diabetes. One patient presented a cerebral spinal fluid fistula, requiring a second approach and reconstruction with a fascia lata graft. In late outcomes, 78% of the patients maintained or improved visual acuity; regarding headache, associated pharmacological therapy allowed the improvement or the stability of 85% of patients [
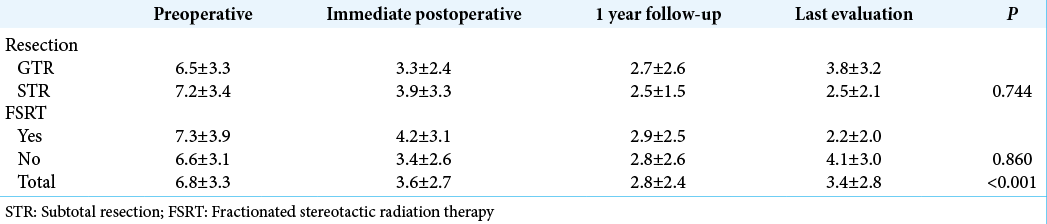
The radiological evaluation had a median follow-up of 39 months. Proptosis significantly improved after surgery and along the follow-up [P < 0.001,
Ten patients presented recurrence/regrowing and were submitted to adjuvant FSRT, six of them after an STR. All patients of this subgroup had proptosis. It was observed a higher frequency of worse in visual acuity in patients submitted to FSRT [71% vs. 28%, P = 0.038, Table 1]. It was observed proptosis improvement (quantified in imaging) in the FSRT group compared with other patients [2.2 ± 2.0 mm vs. 4.1 ± 3.0 mm, P = 0.147]. Until now, these patients are still with controlled disease.
DISCUSSION
Like other meningiomas, SOM is mostly benign tumors which exhibit a remarkable bone invasion, associated with a dural en-plaque growth. This morphology, related to the proximity of delicate structures, hinders the total resection of these lesions and increases recurrence/regrowth.[
Due to the 11 patients (27.5%) who underwent the first resection in other services and were reoperated in this series, we preferred to evaluate clinical follow-up and quantify proptosis rather than stratify individuals according to tumor recurrence. We observed around one-third of STR in our service. Although it was expected a relatively worse outcome in the STR group, it had a slightly lower quantifiable proptosis in the last evaluation than the GTR group (2.5 ± 2.1 mm vs. 3.8 ± 3.2 mm). This finding suggests even an incomplete removal of the tumor can provide good late outcomes.
Ten patients underwent FSRT and EOR influenced adjuvant treatment since six of these patients had an STR. In addition, this group presented a higher prevalence of visual acuity worsening in the last follow-up. This finding is associated with a worse preoperative setting in the FSRT group, since nine of the 10 patients submitted to FSRT presented preoperative decrease visual acuity, against 56% of the patients without FSRT. Nonetheless, patients submitted to STR associated with adjuvant FSRT had lower proptosis in the late outcome.
There was a high prevalence of proptosis (90%) in our population, similar to the previous case series.[
The surgical technique involves the pretemporal craniotomy, to remove all the bone and tumor from the temporal pole until the foramen rotundum, and associate the superolateral orbitotomy, allowing orbital decompression and the removal of the intraorbital tumor. There are many approaches for this tumor. As discussed in a recent meta-analysis,[
Reconstruction of orbital walls and the lesser or greater sphenoid wings, especially with autologous bone graft, has been endorsed by some authors. The main advantages reported are the esthetic improvement and prevention of pulsating exophthalmos, oculomotor muscle fibrosis, meningoceles, and enophthalmos.[
There are some limitations needed to be addressed. We assessed clinical data retrospectively. In addition, only 10 patients underwent radiotherapy (25%), hindering the analysis of radiotherapy effect on SOM recurrence. Finally, there was a high rate of follow-up withdrawal, compromising data about late outcomes. This is a case series and just shows a tendency for exophthalmos improvement without enophthalmos in patients who did not underwent orbital reconstruction. More studies are necessary to give more significance for these findings, including randomized clinical trials.
CONCLUSION
Although a demanding procedure, surgical resection of SOM was effective in stopping the evolution of visual deficit and improvement of proptosis. Adjuvant RT was reserved for patients with STR and worst visual acuity. The nonreconstruction of the orbit does not seem to correlate with a higher prevalence of enophthalmos. Most of the perioperative complications were solved during the hospital stay and did not affect late clinical outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amirjamshidi A, Abbasioun K, Amiri RS, Ardalan A, Hashemi SM. Lateral orbitotomy approach for removing hyperostosing en plaque sphenoid wing meningiomas. Description of surgical strategy and analysis of findings in a series of 88 patients with long-term follow up. Surg Neurol Int. 2015. 6: 79
2. Baucher G, Troude L, Roux A, Loundou A, Boucekine M, Meling T. Predictors of visual function after resection of skull base meningiomas with extradural anterior clinoidectomy. Neurosurg Rev. 2022. 45: 2133-49
3. Boari N, Gagliardi F, Spina A, Bailo M, Franzin A, Mortini P. Management of spheno-orbital en plaque meningiomas: Clinical outcome in a consecutive series of 40 patients. Br J Neurosurg. 2013. 27: 84-90
4. Brusati R, Biglioli F, Mortini P, Raffaini M, Goisis M. Reconstruction of the orbital walls in surgery of the skull base for benign neoplasms. Int J Oral Maxillofac Surg. 2000. 29: 325-30
5. Chambless LB, Mawn LA, Forbes JA, Thompson RC. Porous polyethylene implant reconstruction of the orbit after resection of spheno-orbital meningiomas: A novel technique. J Craniomaxillofac Surg. 2012. 40: e28-32
6. Cushing H. The cranial hyperostosis produced by meningeal endotheliomas. Arch Neurol Psychiatry. 1922. 8: 139-54
7. Cushing H, Eisenhardt L. The Meningiomas: Their Classification, Regional Behavior, Life History, and Surgical end Results. Vol. Toronto: Springer Charles C Thomas; 1938. 27: 185
8. Fisher FL, Zamanipoor Najafabadi AH, Schoones JW, Genders SW, van Furth WR. Surgery as a safe and effective treatment option for spheno-orbital meningioma: A systematic review and meta-analysis of surgical techniques and outcomes. Acta Ophthalmol. 2021. 99: 26-36
9. Güdük M, Özduman K, Pamir MN. Sphenoid wing meningiomas: Surgical outcomes in a series of 141 cases and proposal of a scoring system predicting extent of resection. World Neurosurg. 2019. 125: e48-59
10. Heufelder MJ, Sterker I, Trantakis C, Schneider JP, Meixensberger J, Hemprich A. Reconstructive and ophthalmologic outcomes following resection of spheno-orbital meningiomas. Ophthalmic Plast Reconstr Surg. 2009. 25: 223-6
11. Honig S, Trantakis C, Frerich B, Sterker I, Schober R, Meixensberger J. Spheno-orbital meningiomas: Outcome after microsurgical treatment: A clinical review of 30 cases. Neurol Res. 2010. 32: 314-25
12. Idowu OO, Ashraf DC, Magill ST, Kersten RC, McDermott MW, Vagefi MR. Multidisciplinary frontotemporal orbitozygomatic craniotomy for spheno-orbital meningiomas: Ophthalmic and orbital outcomes. Ophthalmic Plast Reconstr Surg. 2021. 37: 18-26
13. Ko CC, Lim SW, Chen TY, Chen JH, Li CF, Shiue YL. Prediction of progression in skull base meningiomas: Additional benefits of apparent diffusion coefficient value. J Neurooncol. 2018. 138: 63-71
14. Leroy HA, Leroy-Ciocanea CI, Baroncini M, Bourgeois P, Pellerin P, Labreuche J. Internal and external spheno-orbital meningioma varieties: Different outcomes and prognoses. Acta Neurochir (Wien). 2016. 158: 1587-96
15. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
16. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
17. Mariniello G, Maiuri F, Strianese D, Donzelli R, Iuliano A, Tranfa F. Spheno-orbital meningiomas: Surgical approaches and outcome according to the intraorbital tumor extent. Zentralbl Neurochir. 2008. 69: 175-81
18. Mariniello G, de Divitiis O, Bonavolontà G, Maiuri F. Surgical unroofing of the optic canal and visual outcome in basal meningiomas. Acta Neurochir (Wien). 2013. 155: 77-84
19. Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L. Recurrent spheno-orbital meningioma. J Neurosurg. 1994. 80: 202-8
20. Maugeri R, Graziano F, Basile L, Gulì C, Giugno A, Giammalva GR, Visocchi M, Mehdorn HM, Katayama Y, von Wild KR.editors. Trends in Reconstructive Neurosurgery. Cham. Springer International Publishing; 2017. p.
21. Mourits MP, van der Sprenkel JW. Orbital meningioma, the Utrecht experience. Orbit. 2001. 20: 25-33
22. Nagahama A, Goto T, Nagm A, Tanoue Y, Watanabe Y, Arima H. Spheno-orbital meningioma: Surgical outcomes and management of recurrence. World Neurosurg. 2019. 126: e679-87
23. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019. 21: v1-100
24. Pompili A, Derome PJ, Visot A, Guiot G. Hyperostosing meningiomas of the sphenoid ridge clinical features, surgical therapy, and long-term observations: Review of 49 cases. Surg Neurol. 1982. 17: 411-6
25. Ringel F, Cedzich C, Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007. 60: 214-21
26. Saeed P, van Furth WR, Tanck M, Kooremans F, Freling N, Streekstra GI. Natural history of spheno-orbital meningiomas. Acta Neurochir (Wien). 2011. 153: 395-402
27. Sandalcioglu IE, Gasser T, Mohr C, Stolke D, Wiedemayer H. Spheno-orbital meningiomas: Interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005. 33: 260-6
28. Scarone P, Leclerq D, Héran F, Robert G. Long-term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. Clinical article. J Neurosurg. 2009. 111: 1069-77
29. Shrivastava RK, Sen C, Costantino PD, Della Rocca R. Sphenoorbital meningiomas: Surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005. 103: 491-7
30. Simpson D. The recurrence of intracranlal meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957. 20: 22-39
31. Talacchi A, De Carlo A, D’Agostino A, Nocini P. Surgical management of ocular symptoms in spheno-orbital meningiomas. Is orbital reconstruction really necessary? Neurosurg Rev 2014. ;. 37: 301-9
32. Terrier LM, Bernard F, Fournier HD, Morandi X, Velut S, Hénaux PL. Spheno-orbital meningiomas surgery: Multicenter management study for complex extensive tumors. World Neurosurg. 2018. 112: e145-56