- Department of Stem Cell Biology, School of Medicine, Konkuk University, Seoul, Korea
- Department of Laboratory Animal Medicine, College of Veterinary Medicine, Seoul National University, Seoul, Korea
- Department of Physiology, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
- Department of Chemistry, Rice University, Houston, Texas, USA
- The NanoCarbon Center, Rice University, Houston, Texas, USA
- Department of Material Science and Nanoengineering, Rice University, Houston, Texas, USA
Correspondence Address:
Bae Hwan Lee, James M. Tour
Department of Chemistry, Rice University, Houston, Texas, USA
The NanoCarbon Center, Rice University, Houston, Texas, USA
Department of Material Science and Nanoengineering, Rice University, Houston, Texas, USA
DOI:10.4103/2152-7806.190475
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kim C, Sikkema WKA, Hwang I, Oh H, Kim UJ, Lee BH, Tour JM. Spinal cord fusion with PEG-GNRs (TexasPEG): Neurophysiological recovery in 24 hours in rats. Surg Neurol Int 13-Sep-2016;7:
How to cite this URL: Kim C, Sikkema WKA, Hwang I, Oh H, Kim UJ, Lee BH, Tour JM. Spinal cord fusion with PEG-GNRs (TexasPEG): Neurophysiological recovery in 24 hours in rats. Surg Neurol Int 13-Sep-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/spinal-cord-fusion-peg%e2%80%91gnrs-texaspeg-neurophysiological-recovery-24-hours-rats/
Abstract
Background:The GEMINI spinal cord fusion protocol has been developed to achieve a successful cephalosomatic anastomosis. Here, for the first time, we report the effects of locally applied water-soluble, conductive PEG(polyethylene glycol)ylated graphene nanoribbons (PEG-GNRs) on neurophysiologic conduction after sharp cervical cord transection in rats. PEG-GNRs were produced by the polymerization of ethylene oxide from anion-edged graphene nanoribbons. These combine the fusogenic potential of PEG with the electrical conducting properties of the graphene nanoribbons.
Methods:Laminectomy and transection of cervical spinal cord (C5) was performed on Female Sprague-Dawley (SD) rats. After applying PEG-GNR on the severed part, electrophysiological recovery of the reconstructed cervical spinal cord was confirmed by somatosensory evoked potentials (SSEPs) at 24 h after surgery.
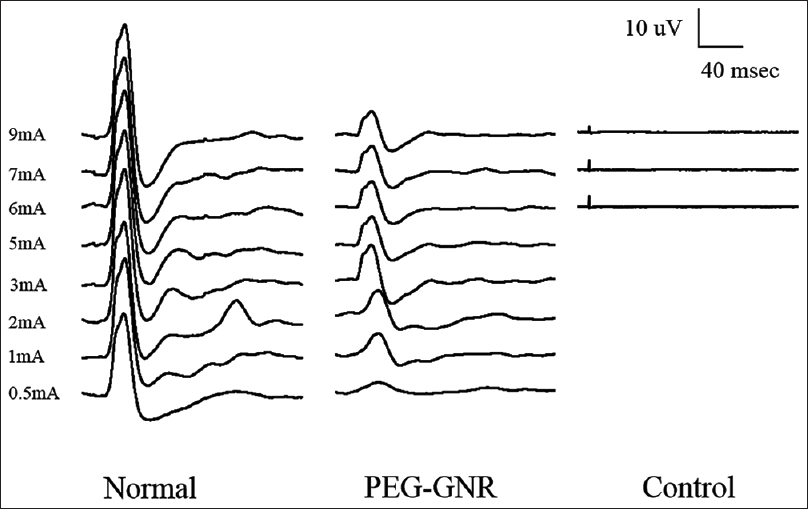
Results:While no SSEPs were detected in the control group, PEG-GNR treated group showed fast recovery of SSEPs at 24 h after the surgery.
Conclusion:In this preliminary dataset, for the first time, we report the effect of a novel form of PEG with the goal of rapid reconstruction of a sharply severed spinal cord.
Keywords: Cephalosomatic anastomosis, electrophysiology, graphene nanoribbons, GEMINI, spinal cord fusion
INTRODUCTION
To achieve a successful spinal cord fusion as required during cephalosomatic anastomosis (CSA), an effective technique to assure rapid reinnervation of the body across the divided cervical spinal cord is necessary.[
In the proposed spinal cord fusion protocol called GEMINI, reapposition of two sharply severed cords brings in contact the gray matter cores in which the cortico-truncoreticulo-propriospinal (CTRPS) pathway courses; this cellular core re-establishes contact by regrowth of the severed connections among propriospinal cells and by acute “fusion” of the neural membranes that were transected acutely. This regrowth has been confirmed histologically in animal models of spinal cord sections by Freeman (see in reference 6), who demonstrated a huge amount of fibers regrowing across the stumps interface, and more recently by other groups (see in 3-4,6). The CTRPS pathway – rather than the myelinated fibers of the descending and ascending tracts – is truly responsible for motor and coarse sensory transmission and is the linchpin of the GEMINI spinal cord fusion protocol (for an in-depth review of the key role of the evolutionarily conserved CTRPS in sensorimotor integration, refer to references 3–6).
The GEMINI fusion process achieves accelerated fiber fusion by way of substances called fusogens.[
Despite the properties of PEG having been discovered in 1986, very little work has been published over the subsequent 30 years.[
Fusogens exert their beneficial effect maximally when applied locally to the point of transection rather than when administered parenterally.[
In light of these data, we asked whether results could be improved by adding conductive, high-aspect ratio graphene nanoribbons (GNR) to the fusogen solution. In a three-dimensional tissue setting, across a gap in the spinal cord, we expected the GNRs to first act as an electrical conduit and then act as an electrically active scaffold upon which the neurons will grow, directing their processes in the proper direction across the gap. We expect this result primarily because GNRs have been patterned on two-dimensional surfaces cause growing and differentiating neurons to take on the same pattern.[
We have recently shown how a sharp transection aided by PEG alone can restore at least partial motor function in a rat and mice[
In this paper, we report for the first time on the effects of a locally applied water-soluble, PEGylated conductive GNR solution on neurophysiologic conduction after sharp cervical cord transection in rats.
MATERIALS AND METHODS
Polyethylene glycol–graphene nanoribbon (TexasPEG)
Multi-walled carbon nanotubes (MWCNTs) were obtained from EMD Merck (produced by Mitsui and Co., lot no. 2699-64E) and were used as received. Tetrahydrofuran (THF) was dried over solid KOH for several days, degassed, and freshly distilled from sodium/benzophenone under a N2 atmosphere. All chemicals were purchased from Sigma-Aldrich unless otherwise specified. Thermogravimetric analysis (TGA) measurements were performed on a TA instruments Q-600 Simultaneous TGA/DSC. The temperature was ramped at 10°C/min until 900°C under Ar. For transmission electron microscopy (TEM) analysis, the PEG-GNRs were dispersed in water and drop cast onto a lacey carbon grid. For scanning electron microscope (SEM) analysis, the PEG-GNRs were dispersed in o-dichlorobenzene, briefly sonicated in a bath sonicator, and deposited on a smooth metal disk, from which the solvent was evaporated on a heat plate at <100°C. The sample was imaged by an FEI Quanta 400 ESEM FEG instrument. One gram of Mitsui MWCNTs was added to a 1 L oven-dried, nitrogen-purged, Schlenk flask; 500 mL of THF was added. Eutectic NaK (2.5 mL) 1:3.3 by mass (1:1.9 by mol) was added under N2. The reaction mixture was stirred at room temperature for 3 days, until very few liquid droplets of NaK remained. The reaction was cooled in a dry ice/acetone bath to −78°C, and 30 g (0.7 mol) of gaseous ethylene oxide was added from a lecture bottle over 90 min. The mixture was slowly brought to room temperature and stirred for 3 days. A mixture of NaH (20 mmol, 0.53 g) and propargyl bromide (20 mmol, 2.4 g) suspended/dissolved in dry toluene was added to terminate the ethylene oxide polymerization. The reaction was quenched by the addition of 20 L of water, and the dark grey precipitate was collected via filtration on a 0.22 μm polyethersulfone (PES) membrane. The dark grey precipitate was filtered through a polytetrafluoroethylene (PTFE) membrane (0.45 μm), followed by crossflow filtration with a 50 kDa MWCO PES filter to remove unbound polymer. The PEG-GNRs final product (1.3 g) was collected on a PTFE membrane (0.45 μm), washed with DI water (3 × 100 mL), ethanol (3 × 100 mL), DI water (3 × 100 mL), and dried under high vacuum overnight. The propargyl units were added to some of the termini for future peptide additions if desired. Prior to use, the PEG-GNRs were dispersed in PEG 600 (0.5 wt% by GNR concentration). The mixture was tightly sealed in a 50 mL conical vial and was sterilized by 120°C pressurized steam for 30 min.[
Surgery
The experiment was carried out in accordance with animal ethics guidelines and was approved by the Institutional Animal Care and Use Committee of Konkuk University (Seoul, South Korea). Female Sprague-Dawley rats (250~280 g, Young Bio, Gyeonggi-do, Korea) were anesthetized using zoletil and xylazine (3:1 ratio, 1 ml/kg). The muscles overlying the cervical vertebral column were reflected exposing C4-6; a C5 laminectomy was performed and the dura mater was split open longitudinally. After gently raising the cervical cord with a hook, full severance was performed with surgical sharp blades #11.
The experimental group (n = 5) was treated with the PEG-GNR solution (0.5 mL per animal) directly applied at the level of transection of the cervical cord. The control group (n = 5) was treated with the same volume of phosphate buffered saline. The muscle and fascia were sutured and the skin closed. Dextrose 5% solution (20 mL/kg) was administered daily via intraperitoneal injection. The two stumps of the spinal cord of the rats were kept in mechanical proximity by simple hyperextension of the head.
Electrophysiology
One day after the surgery, the animals were reanesthetized with urethane (1.25 g/kg, i.p.). Each animal was placed on a stereotaxic device (Narishige Scientific Instrument Laboratory, Tokyo, Japan) and artificially ventilated using a small animal respirator (Model 683, Rodent Ventilator, Harvard, Holliston, MA, USA). Somatosensory evoked potentials (SSEPs) were recorded to measure the conduction recovery of the sensory system. A special electrode (NE-120, Rhodes Medical Instruments, Inc., Woodland Hills, CA, USA) was used for SSEP recording. For the SSEP recording, the recording electrode was placed in the sensorimotor cortex (bregma: −2 mm, lateral: 2 mm; recording side). A bipolar platinum wire electrode placed in the contralateral sciatic nerve (stimulating side) was used as a stimulating electrode.
A single square pulse (0.1 ms duration) of electrical stimulus was delivered by a stimulus isolator (A365D, World Precision Instruments, Inc., New Haven, CT, USA) that was driven by a pulse generator (Pulsemaster A300, World Precision Instruments). The analog signals of the evoked potentials were amplified (×10000), filtered (Band-Pass 300–1000 Hz), and fed to an IBM-compatible PC through an AD/DA converter (CED 1401, Cambridge, UK) to be averaged out using Spike 2 software. SSEP consisted of an average of 100–300 single sweep epochs. The effect of the stimulation intensity on SSEPs was analyzed in the wave forms by latencies and amplitudes.
Behavioral assessment
Four of the PEG-GNR treated rats died accidentally (drowning during a storm that filled the underground lab) subsequently to the SSEP study. The surviving rat is reported here versus controls treated with saline. The modified Basso, Beattie, and Bresnahan (mBBB) 22-point (0–21) scoring was employed. A score of 21 indicates unimpaired locomotion as observed in uninjured animals, whereas a score of 0 indicates complete absence of voluntary movement. Assessment occurred daily for 2 weeks.
RESULTS
SSEPs were used to evaluate the functional integrity of ascending sensory pathways following surgery and topical treatment. SSEPs are a quantitative way to assess the conduction of somatosensory pathways following cervical cord transection. SSEPs were measured from normal rats (without transection) to acquire baseline recordings for comparison.
In the behavioral assessment portion of this study, recovery in the surviving rat was steady. Twenty-four hours after the surgery, weak tremors of the forelimbs were apparent. Two days later, slight voluntary movements of all 4 paws were occasionally present (mBBB score of fore limbs and hind limbs: 4, 4 respectively). At 1 week [
DISCUSSION
In this preliminary report, we show how the conductive GNR additive to PEG[
As made clear elsewhere,[
In our case, in order to mimic the gentle apposition afforded by this connector, PEG-GNRs were infused into the gap left by the sharp transection at C5 in which the two stumps of the spinal cord of rats were kept in mechanical proximity by simple hyperextension of the head.
In this preliminary study, PEG certainly fused a quota of transacted axons, which is responsible for the recovery of SSEPs. However, given the much quicker neurophysiological recovery as compared with a similar PEG-only study that assessed SSEP after full dorsal transection,[
Evidence suggests that molecular weights of PEG of <1000 Daltons may be toxic in humans.[
CONCLUSION
In conclusion, we report for the first time the effect of a novel form of PEG with the goal of rapid reconstruction of a sharply severed spinal cord. Ongoing studies will clarify its full potential.
Financial support and sponsorship
The research was partially supported by the National Research Foundation of Korea (NRF) Grant (2015R1C1A1A02037047).
Conflicts of interest
There are no conflicts of interest.
Video is Availabe on: www.surgicalneurologyint.com
Acknowledgement
The authors thank Prof. Canavero for his invaluable assistance in analyzing the data and coordinating the centers involved in the study.
References
1. Akhavan O, Ghaderi E. Differentiation of human neural stem cells into neural networks on graphene nanogrids. J Mater Chem B. 2013. 1: 6291-301
2. Bauhofer W, Kovacs JZ. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos Sci Technol. 2009. 69: 1486-98
3. Canavero S. Commentary. Surg Neurol Int. 2015. 6: 103-
4. Canavero S. The “Gemini” spinal cord fusion protocol: Reloaded. Surg Neurol Int. 2015. 6: 18-
5. Canavero S. HEAVEN: The head anastomosis venture Project outline for the first human head transplantation with spinal linkage (GEMINI). Surg Neurol Int. 2013. 4: S335-42
6. Canavero S, Ren X, Kim CY, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI). Surgery. 2016. 160: 11-9
7. Kim CY. PEG-assisted reconstruction of the cervical spinal cord in rats: Effects on motor conduction at 1 h. Spinal Cord. 2016. p.
8. Kim ND, Metzger A, Hejazi V, Li Y, Kovalchuk A, Lee SK. Microwave Heating of Functionalized Graphene Nanoribbons in Thermoset Polymers for Wellbore Reinforcement. ACS App Mater Interfaces. 2016. 8: 12985-91
9. Lalwani G, Patel SC, Sitharaman B. Two- and Three-Dimensional All-Carbon Nanomaterial Assemblies for Tissue Engineering and Regenerative Medicine. Ann Biomed Eng. 2016. 44: 2020-35
10. Lu W, Ruan G, Genorio B, Zhu Y, Novosel B, Peng Z. Functionalized graphene nanoribbons via anionic polymerization initiated by alkali metal-intercalated carbon nanotubes. ACS Nano. 2013. 7: 2669-75
11. Ye Y, Kim CY, Miao Q, Ren X. Fusogen-assisted rapid reconstitution of anatomophysiologic continuity of the transected spinal cord. Surgery. 2016. 160: 20-5







Guy Woods
Posted December 18, 2016, 4:21 am
Wonderful to hear your results on Quirks and Quarks today. Our son is a quadriplegic and we live in hope that something like this will restore even some of his mobility. Keep up the great work and we will watch for further developments. Merry Xmas from Nelson, BC, Canada