- Department of Neurosurgery, Kobe University Graduate School of Medicine, Kobe, Japan.
Correspondence Address:
Jun Imura, Department of Neurosurgery, Kobe University Graduate School of Medicine, Kobe, Japan.
DOI:10.25259/SNI_604_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jun Imura, Atsushi Fujita, Subaru Umeda, Yosuke Fujimoto, Masaaki Kohta, Takashi Sasayama. Spinal cord infarction after coil embolization of a basilar tip aneurysm: A case report and literature review. 09-Sep-2022;13:415
How to cite this URL: Jun Imura, Atsushi Fujita, Subaru Umeda, Yosuke Fujimoto, Masaaki Kohta, Takashi Sasayama. Spinal cord infarction after coil embolization of a basilar tip aneurysm: A case report and literature review. 09-Sep-2022;13:415. Available from: https://surgicalneurologyint.com/surgicalint-articles/11851/
Abstract
Background: Spinal cord infarction is a rare but serious complication of neurointervention that has been rarely documented. An association between spinal cord infarction and the placement of large bore catheters in the vertebral artery (VA) has been mentioned, but the precise etiology remains unclear.
Case Description: A 72-year-old female presented with the right hemiparesis and left thermal hypoalgesia directly after endovascular coil embolization for an unruptured basilar tip aneurysm. Magnetic resonance imaging demonstrated right-sided upper cervical (C2-3) spinal cord infarction. Conventional bilateral vertebral angiograms revealed no opacification of the anterior spinal arteries. Cone-beam computed tomography showed a watershed area of radiculomedullary arteries that was correlated with the extent of the ischemic lesion. Thus, the spinal cord ischemia may have had multifactorial causes combined with reduced perfusion pressure to the spinal cord, which was caused by the placement of the guiding catheter in the VA and intensive hypotension during general anesthesia.
Conclusion: Spinal cord infarction should be recognized as a serious complication of endovascular treatment involving posterior circulation.
Keywords: Coil embolization, Complication, Intracranial aneurysm, Posterior circulation, Spinal cord infarction
INTRODUCTION
Spinal cord infarction is a rare but serious complication of neurointerventional procedures, but its etiology has been rarely recognized. It is a severe complication that requires awareness because it can significantly impact the functional outcome of patients. To the best of our knowledge, spinal cord infarction has only been described in four previous case reports.[
Herein, we report a patient with cervical spinal cord infarction after an elective neurointerventional procedure. The patient presented with Brown-Séquard syndrome after the procedure, and acute ischemia was detected on the right side of the spinal cord using magnetic resonance imaging (MRI). To investigate the etiology of the spinal cord infarction, we performed cone-bean computed tomography (CBCT), which allowed us to precisely identify the angioarchitecture. We discuss the etiology of the spinal cord infarction based on the findings of the CBCT scan and review the literature.
CASE REPORT
A 72-year-old woman with a history of multiple anterior circulation cerebral aneurysm clipping (6 years previously) was referred to our department because of a gradually growing and unruptured basilar tip aneurysm. Her neurological examination was normal and a cerebral angiogram revealed a basilar tip aneurysm with a maximum size of 4.6 mm. Endovascular balloon-assisted coil embolization was performed under general anesthesia. Dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg) was started 2 weeks before the surgery, and cilostazol (200 mg) was orally administered on the day of the treatment. A 6-French (Fr) introducer was placed in the right femoral artery. After systemic heparinization (intravenous injection at 5000 units, followed by continuous intravenous infusion of 1000 units/h), a 6-Fr guiding catheter (Roadmaster STR 90 cm; Goodman, Aichi, Japan) was placed in the right VA. The tip of the guiding catheter was placed in the distal end of the V2 segment and a VA angiogram confirmed no findings of decreasing contrast flow from the arterial to venous phases [
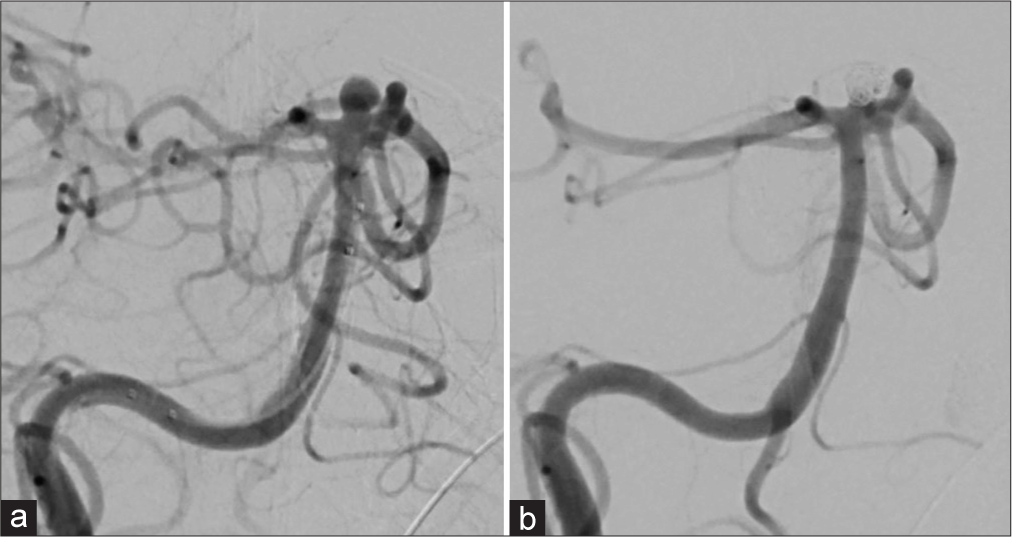
Figure 1:
(a) Anteroposterior view of a right vertebral artery (VA) angiogram shows the unruptured basilar tip aneurysm. The tip of the guiding catheter was not wedged, and there was good flow around the guiding catheter. The anterior spinal artery was not visualized in this angiogram throughout the procedure. (b) Anteroposterior view of a right VA angiogram just after coil embolization shows that the aneurysm was successfully embolized, without signs of reduced VA flow.
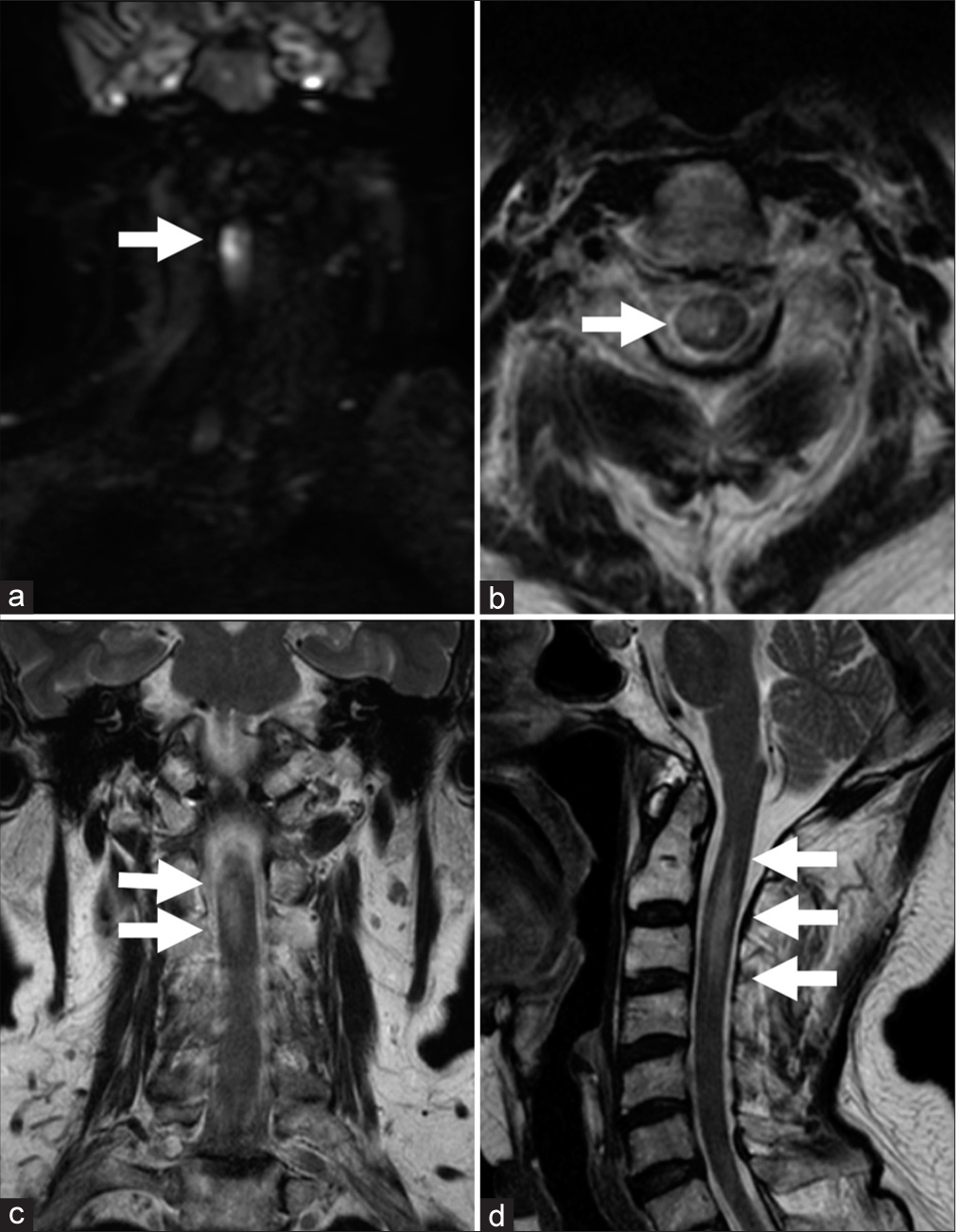
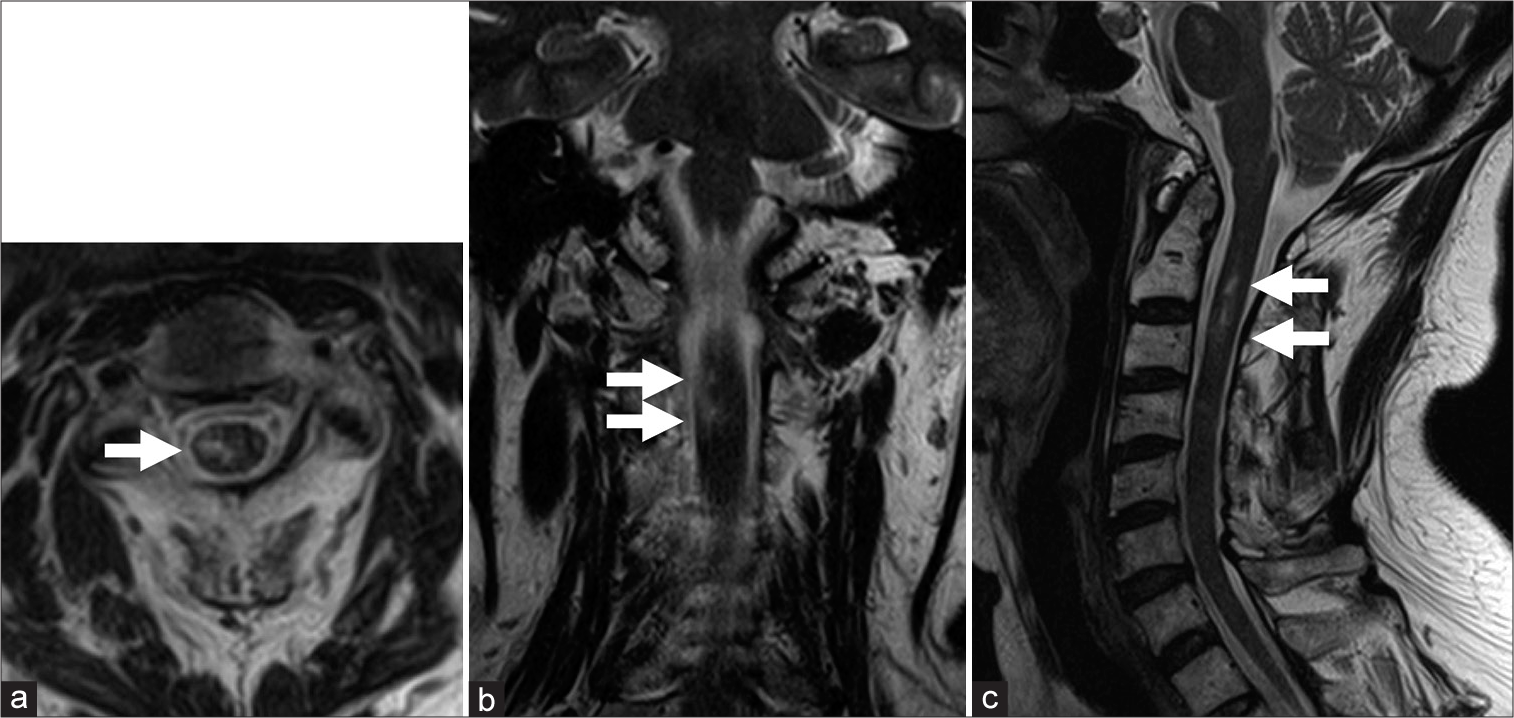
Although the awakening from anesthesia was good, the patient presented with right hemiparesis (Manual Muscle Test: 2/5); thus, an emergency brain MRI was performed. Diffusion-weighted imaging revealed very small ischemic lesions involving the pons and bilateral cerebellar hemispheres; however, these lesions were not related to the patient’s neurological deficit. Four days after the intervention, the patient noted left thermal hypoalgesia, and cervical MRI revealed spinal cord infarction associated with spinal cord swelling on the right side at the level of the second and third cervical vertebral body [
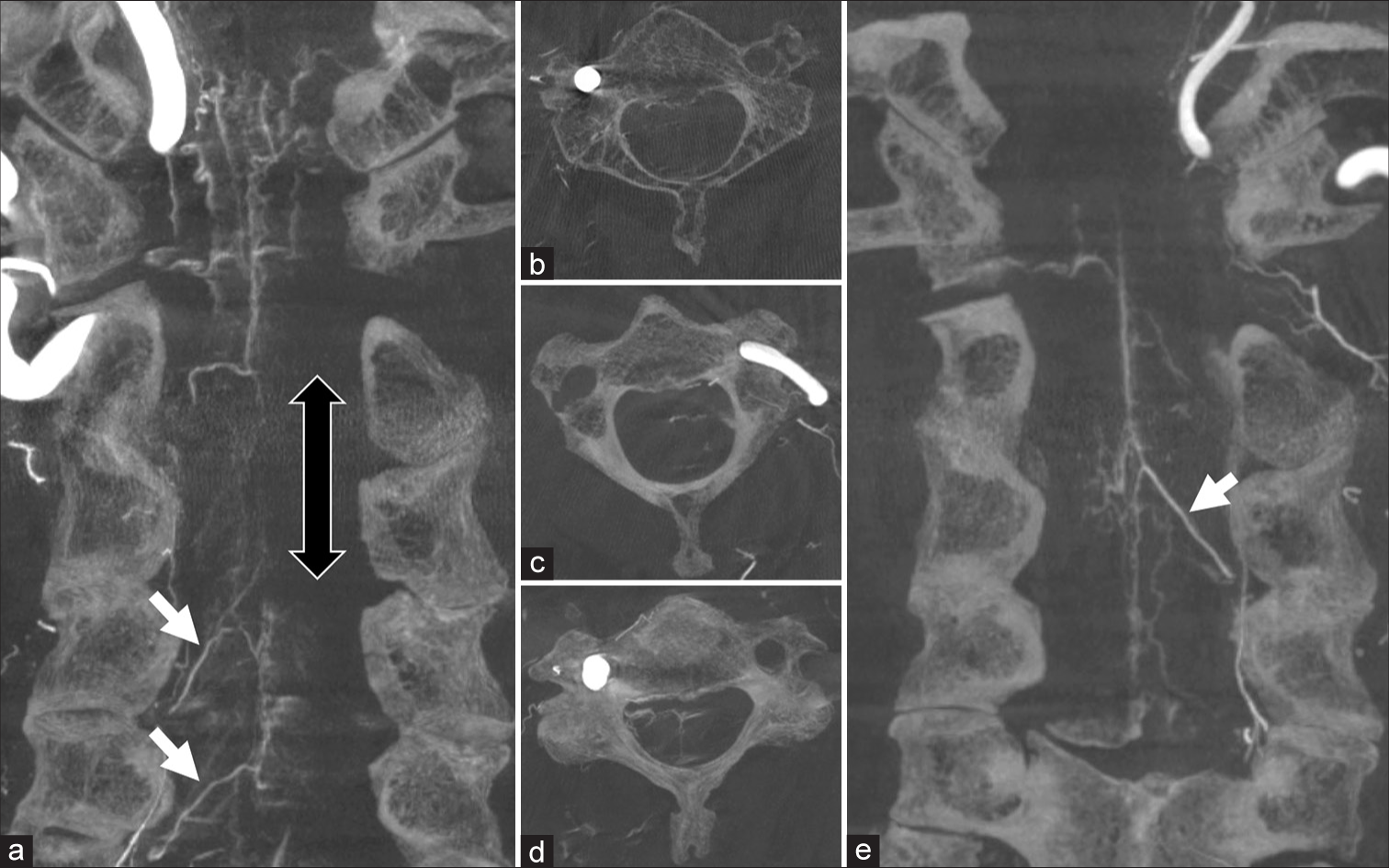
Figure 4:
Reconstructed cone-beam computed tomography (CBCT) scans obtained 4 weeks after coil embolization. A coronal view of the right (a) vertebral artery (VA) shows an avascular area consistent with spinal cord infarction (blank arrow). Arrows indicate radiculomedullary arteries (RMAs) at the level of C4 and C5. An axial CBCT view of the right VA at the level of C3 (b) shows faint opacification around the spinal cord; however, an axial view of the left VA at the same level [Figure 4c] showed clear opacification of the left side of the spinal cord. On the other hand, an axial CBCT view of the right VA at the level of C4 [Figure 4d] showed clear opacification of the bilateral spinal surface pial vessels. A coronal CBCT scan of the left VA showed clear opacification of the RMA [Figure 4e], arrow) connected to an anterior spinal artery.
DISCUSSION
The blood supply of the spinal cord can be separated into a central and a peripheral system. The central system, which supplies two-thirds of the spinal cord, is supplied by the ASA and its branch (the central artery). The peripheral system is supplied by a pair of posterior spinal arteries (PSAs) and the pial artery plexus.[
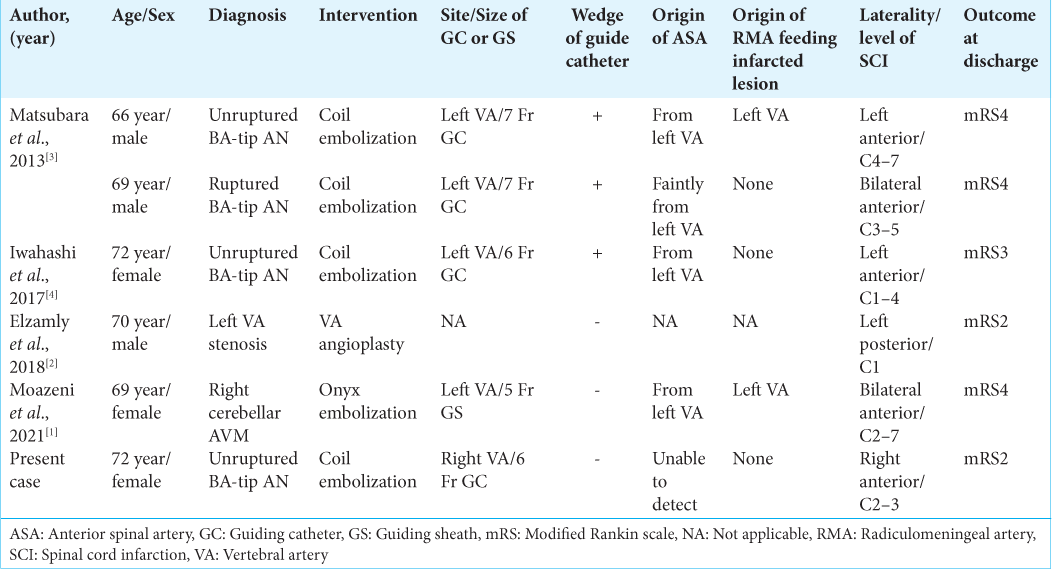
The incidence of spinal cord infarction as a complication of neurointerventional procedures is very rare and has only been described in five case reports.[
In our case, a 6-Fr guiding catheter was not wedged, and there was no flow stagnation in the VA throughout the procedure. As reported in the previous literature, a reduction of VA flow during the procedure is an important risk factor that should be avoided; however, because our patient did not have these factors, we should consider other etiologies. In the present report, we were able to demonstrate the precise angioarchitecture at the subacute stage of spinal cord infarction using CBCT; this has never before been reported. Our CBCT findings revealed that the area of the spinal cord infarction was correlated with a hypovascular watershed area on right VA injection. Although an RMA from the left VA provided blood supply to the ASA at the level of C3, a reconstructed coronal view showed no opacification of the right side of the spinal cord. We speculate that, if a patient has the specific characteristics that we have reported here, spinal cord infarction can occur even in the absence of a catheter wedge or flow reduction.
As previously reported, the cause of the spinal cord infarction will be related to thrombosis of the feeding artery of the spinal cord, adequate antithrombotic therapy was recommended. Routine use of dual antiplatelet therapy and systemic heparization during procedure was mandatory. In cases of coil embolization without stenting, we have used neutralized heparinization with protamine and performed manual compression hemostasis at the femoral puncture site. Considering these cases, the use of femoral vascular closure devices without using protamine should be considered.
Another possible factor related to spinal cord infarction that should be considered is that intraoperative intensive hypotension under general anesthesia may cause a reduction in perfusion pressure to the ASA and RMA. Intensive hypotension also plays an important role in the development of stroke, and a MAP decrease of more than 30% from the baseline has been reported as significantly associated with the occurrence of postoperative stroke.[
CONCLUSION
We reported a patient with cervical spinal cord infarction after the coil embolization of a basilar tip aneurysm and reviewed the recent literature. Our patient’s spinal cord ischemia may have had multifactorial causes combined with reduced perfusion pressure to the spinal cord, which was caused by the placement of the guiding catheter in the VA and intensive hypotension during anesthesia. Spinal cord infarction should be recognized as a serious complication of endovascular treatment involving posterior circulation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bijker JB, Persoon S, Peelen LM, Moons KG, Kalkman CJ, Kappelle LJ. Intraoperative hypotension and perioperative ischemic stroke after general surgery: A nested case-control study. Anesthesiology. 2012. 116: 658-64
2. Crum B, Mokri B, Fulgham J. Spinal manifestations of vertebral artery dissection. Neurology. 2000. 55: 304-6
3. Elzamly K, Nobleza C, Parker E, Sugg R. Unilateral upper cervical posterior spinal cord infarction after a neuroendovascular intervention: A case report. Case Rep Neurol Med. 2018. 2018: 5070712
4. Iwahashi H, Fujita A, Tanaka H, Ikeda M, Morikawa M, Kohmura E. Spinal cord infarction after successful coil embolization of recurrent basilar bifurcation aneurysm: A case report. J Neuroendovasc Ther. 2018. 12: 398-403
5. Machnowska M, Moien-Afshari F, Voll C, Wiebe S. Partial anterior cervical cord infarction following vertebral artery dissection. Can J Neurol Sci. 2008. 35: 674-7
6. Martirosyan NL, Feuerstein JS, Theodore N, Cavalcanti DD, Spetzler RF, Preul MC. Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions: A review. J Neurosurg Spine. 2011. 15: 238-51
7. Matsubara N, Miyachi S, Okamoto T, Izumi T, Asai T, Yamanouchi T. Spinal cord infarction is an unusual complication of intracranial neuroendovascular intervention. Interv Neuroradiol. 2013. 19: 500-5
8. Moazeni Y, Cantrell DR, Clark JR, Abdalla RN, Batra A, Hurley MC. Case report: Anterior spinal cord ischemia following embolization of cerebellar arteriovenous malformation: An illustrative case and review of spinal cord vascular anatomy. Front Neurol. 2021. 12: 725065