- Department of Neurosurgery and Neuroendovascular Therapy, St. Luke’s International Hospital, Tokyo, Japan.
- Department of Clinical Laboratory and Intraoperative Neurophysiology, St. Luke’s International Hospital, Tokyo, Japan.
Correspondence Address:
Shogo Shima, Department of Neurosurgery and Neuroendovascular Therapy, St. Luke’s International Hospital, Tokyo, Japan. shisho@luke.ac.jp
DOI:10.25259/SNI_592_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shogo Shima1, Yasuko Tanaka2, Shinsuke Sato1, Yasunari Niimi1. Spinal epidural arteriovenous fistula with improved sphincter impairment detected by intraoperative neurophysiological monitoring. 26-Aug-2022;13:384
How to cite this URL: Shogo Shima1, Yasuko Tanaka2, Shinsuke Sato1, Yasunari Niimi1. Spinal epidural arteriovenous fistula with improved sphincter impairment detected by intraoperative neurophysiological monitoring. 26-Aug-2022;13:384. Available from: https://surgicalneurologyint.com/surgicalint-articles/11827/
Abstract
Background: A spinal epidural arteriovenous fistula (SEAVF) is a rare type of arteriovenous shunt that occurs mainly in the thoracic or lumbar spine. Patients with SEAVF develop motor/sensory disturbances of the lower extremities and sphincter dysfunction. Among these symptoms, sphincter impairments show less improvement than others, and its relevance to neurophysiological monitoring has not been documented.
Case Description: A 77-year-old woman presented with progressive motor weakness and numbness in the lower extremities and urinary and fecal incontinence. Spinal magnetic resonance imaging showed spinal cord edema in Th5-Th11 and enlarged perimedullary veins. We performed spinal angiography and endovascular treatment under intraoperative neurophysiological monitoring (IOM), including sensory evoked potential (SEP), motor evoked potential (MEP), and bulbocavernosus reflex (BCR) monitoring. Diagnostic angiography revealed a SEAVF with perimedullary venous drainage fed by the left L2 segmental artery. The shunt was completely embolized using N-butyl-2-cyanoacrylate. Although SEP and MEP of the lower legs were recordable during treatment, anal MEP and BCR were not observed. The sphincter symptoms improved 1.5 years after the treatment. Follow-up angiography revealed no shunt recurrence and improved venous congestion. Anal MEP and BCR were detected during angiography, indicating neurophysiological improvement in sphincter function. The prolonged latency of the monitoring suggested a pudendal nerve injury.
Conclusion: This case report first described improvement of the IOM correlated with the functional recovery of sphincters after embolization of a SEAVF. Follow-up neurophysiological monitoring is important to assess the functional recovery of the sphincter.
Keywords: Endovascular treatment, Intraoperative neurophysiological monitoring, Sphincter impairment, Spinal epidural arteriovenous fistula
INTRODUCTION
Spinal epidural arteriovenous fistulas (SEAVFs) are relatively rare spinal vascular malformations that frequently occur in men aged >60 years and are known to be associated with prior trauma or surgical history.[
CASE PRESENTATION
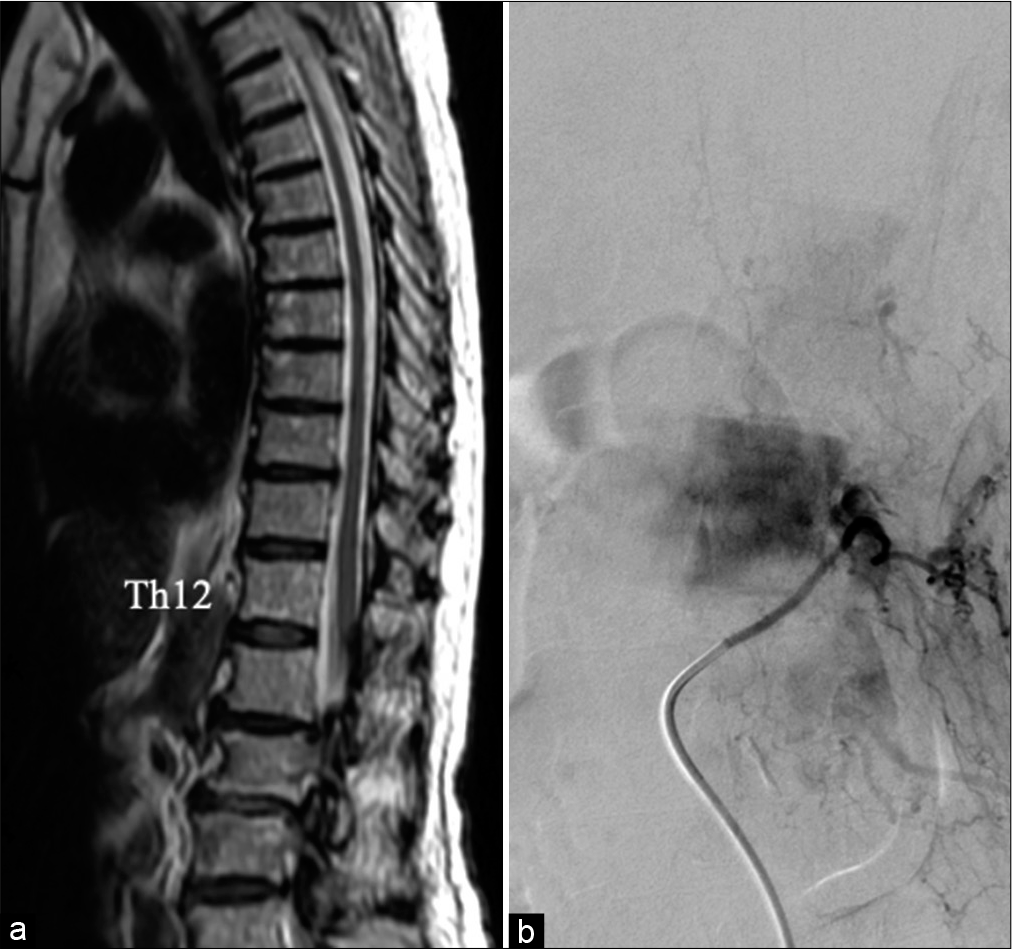
A 72-year-old woman with progressive symptoms of gait disturbance, constipation, and urinary incontinence for 1 year was referred to our hospital. She showed no medical or trauma history and was wheelchair-dependent due to lower-leg weakness. Physical examination revealed motor weakness, bilateral numbness of the lower extremities, and anal sphincter impairment. Her Aminoff-Logue scale gait, micturition, and bowel scores were 5, 2, and 2, respectively. Computed tomography revealed no significant brain abnormalities. Spinal magnetic resonance imaging (MRI) revealed diffuse spinal cord edema in Th5-Th11 and multiple flow voids on the back of the region, indicating venous congestion and perimedullary venous ectasia [
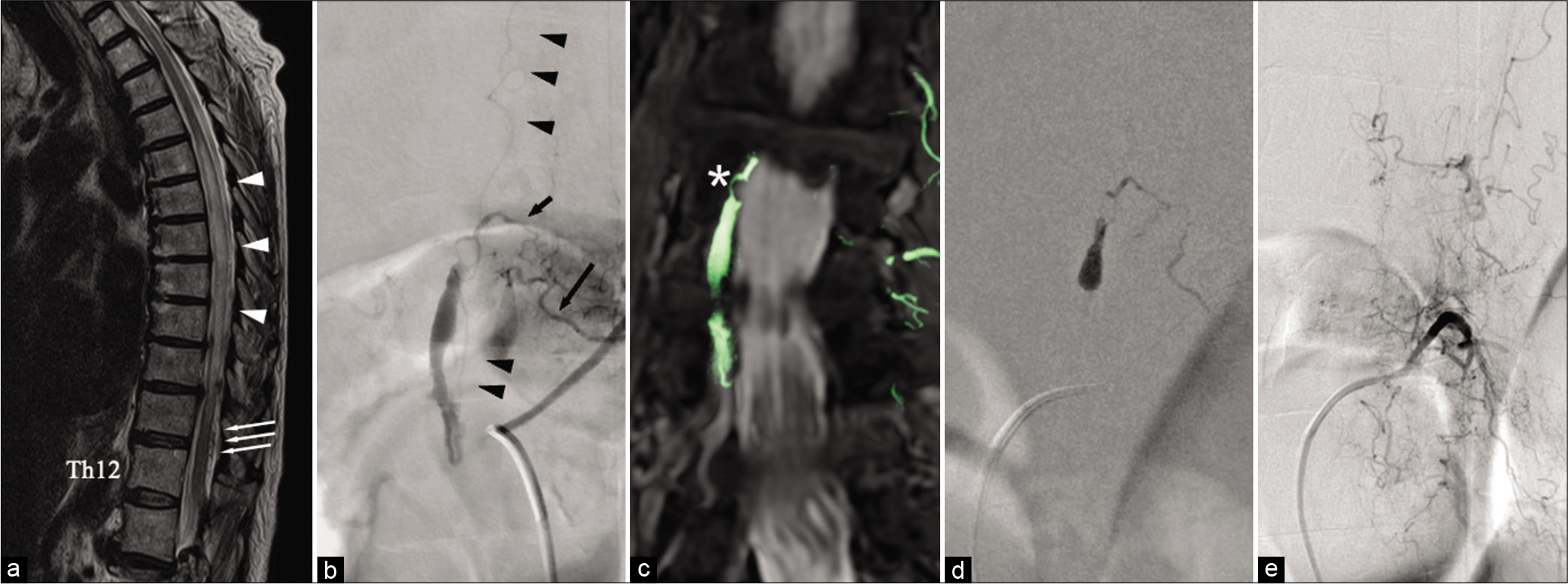
Figure 1:
(a) Sagittal view of T2-weighted magnetic resonance imaging (MRI) of the spinal cord shows a diffuse high signal intensity from Th5 to Th11 (arrowheads) and multiple flow voids in the subarachnoid space (arrows). (b) Preoperative spinal angiography from the left L2 segmental artery reveals a spinal epidural fistula supplied from the dorsal somatic branch (long arrow) through retrocorporeal anastomosis (short arrow). The shunt forms a venous pouch and cranially drains through the posterior spinal vein (arrowheads). (c) The fusion image of MRI and maximum intensity projection shows the shunt point located in the lateral epidural space (asterisk). (d) NBCA injected from the microcatheter infiltrates into the shunt points and upper part of the venous pouch. (e) Postoperative angiography shows complete obliteration of the shunts with remnant venous congestion.
Neurophysiological monitoring
A neurophysiological monitoring unit (Neuromaster MEE-1208; NIHON KOHDEN Corp. Tokyo, Japan) was used for IOM. All electrodes were inserted into the scalp according to the International 10-20 system for electroencephalography. SEPs were elicited by stimulation of the anterior tibial nerve at the ankle (intensity: 20–40 mA, duration: 0.2 ms, and repetition rate: 5.7 Hz) and recorded through needle electrodes inserted into the scalp at CZ’-FZ. MEPs were elicited by transcranial electrical stimulation using corkscrew-like electrodes placed on the scalp at C3-C4. Monophasic stimulation of five square-wave pulses (intensity, 550V; single pulse duration, 0.5 ms; interstimulus interval, 2 ms; and repetition rate, 2 Hz) was applied. MEPs of the lower extremities were recorded using needle electrodes inserted into the abductor pollicis brevis, tibialis anterior, and abductor hallucis. Anal MEPs were recorded using electrodes inserted into the bilateral bulbospongiosus muscles. The stimulating electrode of the BCR was placed at the pubic symphysis, with the cathode on the dorsum of the clitoris and the anode on the labia majora. Short trains of five square-wave pulses (intensity, 40 mA; single pulse duration, 0.2 ms; interstimulus interval, 2 ms; and repetition rate, 2.7 Hz) were used to elicit BCR. Recordings of the evoked response of the BCR were performed using the same needle electrodes as the anal MEPs.
Endovascular treatment and IOM
All spinal angiography and treatments were performed under general anesthesia using propofol (4–10 mg/kg/h) and remifentanil (0.25–0.5 μg/kg/min). Baseline monitoring was performed after induction of general anesthesia. All SEPs and MEPs were recorded, except the anal MEP and BCR at baseline. Diagnostic and treatment angiography was performed using a cobra-shaped 4-French catheter (Medikit, Tokyo, Japan) through the femoral artery access. The left Th8 segmental artery injection demonstrated delayed spinal venous drainage in the anterior spinal artery, while the left L2 segmental artery injection revealed a SEAVF with perimedullary venous drainage. The shunt was supplied from the left dorsal somatic branch through the retrocorporeal anastomosis and was located in the contralateral ventral epidural space with a venous pouch [
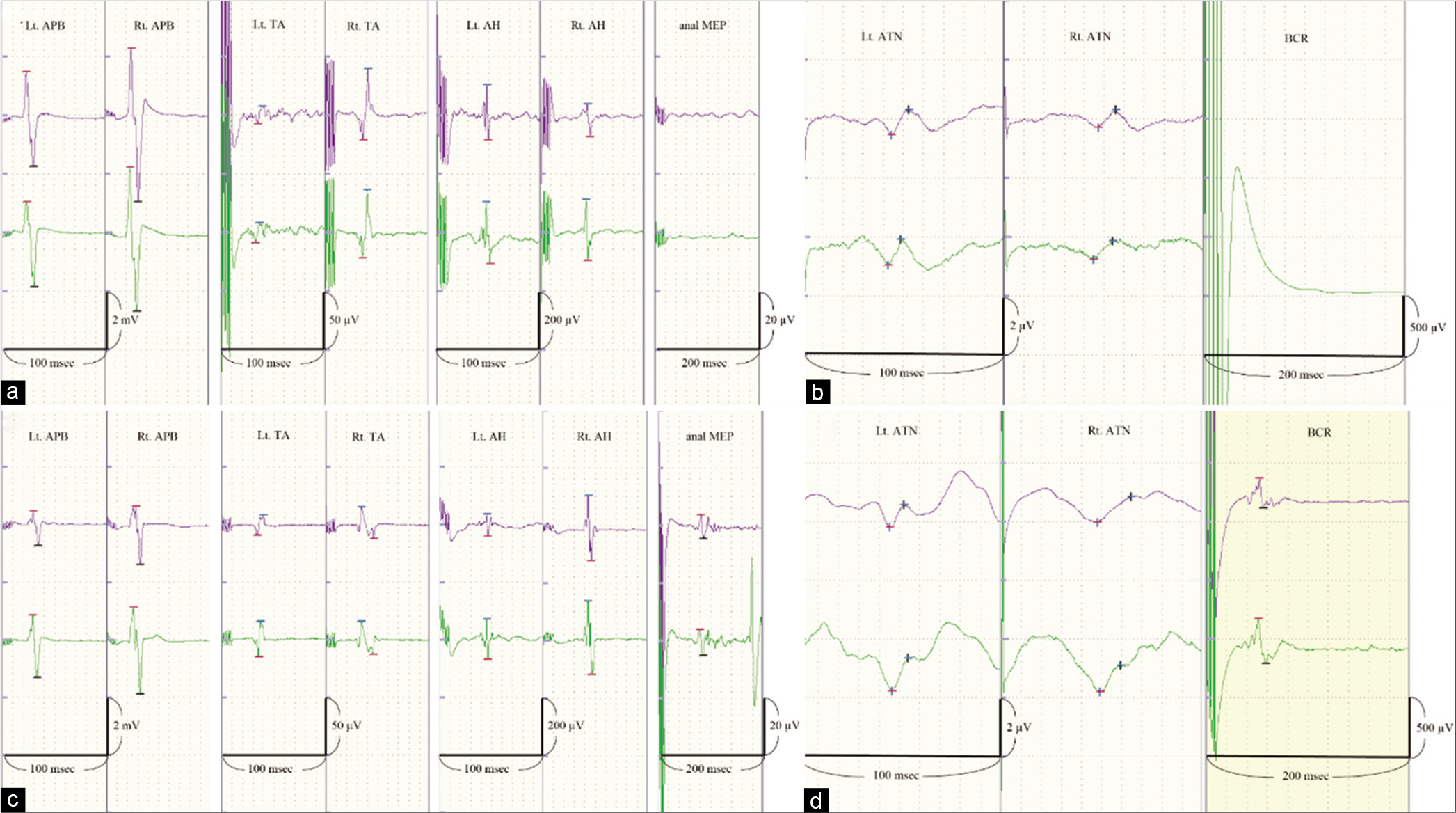
Figure 2:
Potentials in the upper row show baseline records. Potentials in the lower row were recorded at the end of the procedures. (a) Motor evoked potentials (MEPs) from both lower extremities and the anal MEP were monitored at the first treatment. The amplitude and latency of the MEPs from the lower extremities were stable during the embolization of the shunt. The anal MEP was not recorded. (b) The bilateral anterior tibial nerve SEP at the first treatment shows no changes throughout the treatment. The BCR was not recordable throughout the procedure. (c) The anal MEP was observed at the 1.5-year follow-up angiography. The mean amplitude was 8.8 μV, and the mean latency was approximately 80 ms. (d) The BCR was observed at the 1.5-year follow-up angiography. The mean amplitude was 294.9 μV and the mean latency was approximately 50 ms. APB: Abductor pollicis brevis, TA: Tibialis anterior, AH: Abductor hallucis, MEP: Motor evoked potential, SEP: Somatosensory evoked potential, BCR: Bulbocavernosus reflex.
Clinical course
The post treatment clinical course was stable without complications and anticoagulant medication was initiated to prevent venous thrombosis. The patient was transferred to a rehabilitation hospital with a modified Rankin scale score of 3. At the 3-month follow-up, the lower-extremity paraplegia had improved, but fecal and urinary disturbances remained unchanged. Spinal MRI showed the disappearance of flow voids and remnant thoracic cord edema. At 18 months after the treatment, she could walk with a walker, and constipation and urination improved. The Aminoff-Logue scale gait, micturition, and bowel scores were 4, 1, and 1. Anal MEP/BCR monitoring revealed potentials that were not observed during the follow-up angiography [
DISCUSSION
IOM for spinal cord surgery was introduced in the 90s and has been applied to spinal endovascular treatment.[
SEAVF is a rare spinal arteriovenous shunt, which has been lately defined and distinguished from spinal dural arteriovenous fistula (SDAVF) through the recent neuroimaging and angiographic advances.[
The symptoms of spinal vascular shunts, especially sensory and bowel disturbances, tend to be refractory to treatment.[
IOM for spinal arteriovenous shunts can help prevent neurological complications and predict treatment outcomes.[
CONCLUSION
We report a rare case of SEAVF treated with the endovascular approach in which improved sphincter impairment was detected by MEP/BCR monitoring. This case highlights the utility of follow-up neurophysiological monitoring to assess the functional recovery of the sphincter.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Byun JS, Tsang AC, Hilditch CA, Nicholson P, Fang YB, Krings T. Presentation and outcomes of patients with thoracic and lumbosacral spinal epidural arteriovenous fistulas: A systematic review and meta-analysis. J Neurointerv Surg. 2019. 11: 95-8
2. Cenzato M, Debernardi A, Stefini R, D’Aliberti G, Piparo M, Talamonti G. Spinal dural arteriovenous fistulas: Outcome and prognostic factors. Neurosurg Focus. 2012. 32: E11
3. Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: A review focus on the corticospinal tracts. Clin Neurophysiol. 2008. 119: 248-64
4. Granata G, Padua L, Rossi F, De Franco P, Coraci D, Rossi V. Electrophysiological study of the bulbocavernosus reflex: Normative data. Funct Neurol. 2013. 28: 293-5
5. Inoue S, Kawaguchi M, Takashi S, Kakimoto M, Sakamoto T, Kitaguchi K. Intraoperative monitoring of myogenic motor-evoked potentials from the external anal sphincter muscle to transcranial electrical stimulation. Spine (Phila Pa 1976). 2002. 27: E454-9
6. Kaneko K, Taguchi T, Morita H, Yonemura H, Fujimoto H, Kawai S. Mechanism of prolonged central motor conduction time in compressive cervical myelopathy. Clin Neurophysiol. 2001. 112: 1035-40
7. Khaldi A, Hacein-Bey L, Origitano TC. Spinal epidural arteriovenous fistula with late onset perimedullary venous hypertension after lumbar surgery: Case report and discussion of the pathophysiology. Spine (Phila Pa 1976). 2009. 34: E775-9
8. Kiyosue H, Matsumaru Y, Niimi Y, Takai K, Ishiguro T, Hiramatsu M. Angiographic and clinical characteristics of thoracolumbar spinal epidural and dural arteriovenous fistulas. Stroke. 2017. 48: 3215-22
9. Kothbauer K, Pryor JC, Berenstein A, Setton A, Deletis V. Motor evoked potentials predicting early recovery from paraparesis after embolisation of a spinal dural arteriovenous fistula. Interv Neuroradiol. 1998. 4: 81-4
10. Mull M, Othman A, Dafotakis M, Hans FJ, Schubert GA, Jablawi F. Spinal epidural arteriovenous fistula with perimedullary venous reflux: Clinical and neuroradiologic features of an underestimated vascular disorder. AJNR Am J Neuroradiol. 2018. 39: 2095-102
11. Niimi Y, Sala F, Deletis V, Berenstein A. Provocative testing for embolization of spinal cord AVMs. Interv Neuroradiol. 2000. 6: 191-4
12. Niu X, Wang X, Huang H, Ni P, Lin Y, Shao B. Bulbocavernosus reflex test for diagnosis of pudendal nerve injury in female patients with diabetic neurogenic bladder. Aging Dis. 2016. 7: 715-20
13. Sala F, Beltramello A, Gerosa M. Neuroprotective role of neurophysiological monitoring during endovascular procedures in the brain and spinal cord. Neurophysiol Clin. 2007. 37: 415-21
14. Sala F, Niimi Y, Berenstein A, Deletis V. Role of multimodality intraoperative neurophysiological monitoring during embolisation of a spinal cord arteriovenous malformation. A paradigmatic case. Interv Neuroradiol. 2000. 6: 223-34
15. Silva N, Januel AC, Tall P, Cognard C. Spinal epidural arteriovenous fistulas associated with progressive myelopathy. Report of four cases. J Neurosurg Spine. 2007. 6: 552-8