- Department of Neurosurgery, All India Institute of Medical Sciences, Rishikesh, Uttarakhand,
- Department of Neurosurgery, SDM College of Medical Sciences and Hospital, Dharwad, Karnataka,
- Department of Neurosurgery, All India Institute of Medical Science, Raipur, Chhattisgarh, India.
Correspondence Address:
Jitender Chaturvedi
Department of Neurosurgery, All India Institute of Medical Science, Raipur, Chhattisgarh, India.
DOI:10.25259/SNI_545_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jitender Chaturvedi1, Punit Singh1, Sunil Malagi2, Nishant Goyal1, Anil Kumar Sharma3. Spinal extradural arachnoid cyst: Rare cases from Indian Institutes. 25-Sep-2020;11:306
How to cite this URL: Jitender Chaturvedi1, Punit Singh1, Sunil Malagi2, Nishant Goyal1, Anil Kumar Sharma3. Spinal extradural arachnoid cyst: Rare cases from Indian Institutes. 25-Sep-2020;11:306. Available from: https://surgicalneurologyint.com/surgicalint-articles/10287/
Abstract
Background: Spinal extradural arachnoid cyst (SEDAC), accounting for approximately 1% of all spinal lesions, rarely causes compressive myelopathy. It is usually found at lower thoracic or upper lumbar levels in males in their forties to sixties. The standard surgical procedures include direct dural repair.
Case Description: A 37-year-old male presented with myelopathy attributed to a type I meningeal cyst (SEDAC) that was successfully managed with a laminectomy, cyst excision, and direct dural sleeve repair. Similar cases reported in the literature were also reviewed.
Conclusion: SEDACs, although rare, must be considered among the differential diagnoses for compressive myelopathy/neurogenic bladder.
Keywords: Arachnoid cyst, Compressive myelopathy, Extradural, India, Meningeal cyst, Spine
INTRODUCTION
Spinal extradural arachnoid cyst (SEDAC) is a rare cause of compressive myelopathy, accounting for approximately 1% of all spinal lesions.[
Here, along with a literature review, we present a 37-year-old male with a SEDAC successfully managed with a laminectomy, excision of cyst, and defective dural sleeve repair.
CASE REPORT
A 37-year-old male presented with progressive lower extremity paraparesis of 3 years duration (motor power of 3/5 in both legs with a 0/5 left foot drop for 1 year). There were no history of back pain, significant trauma, spinal surgery/lumbar puncture, or tuberculosis.
The thoracic MR showed, on axial cuts, an arachnoid cyst with lateral extension into both neural foramina between the T12-L1 levels; no further lesions were noted on the cervical or lumbar MR studies.
Surgery
The patient underwent L1 preserving laminectomies from T11 to L2 [
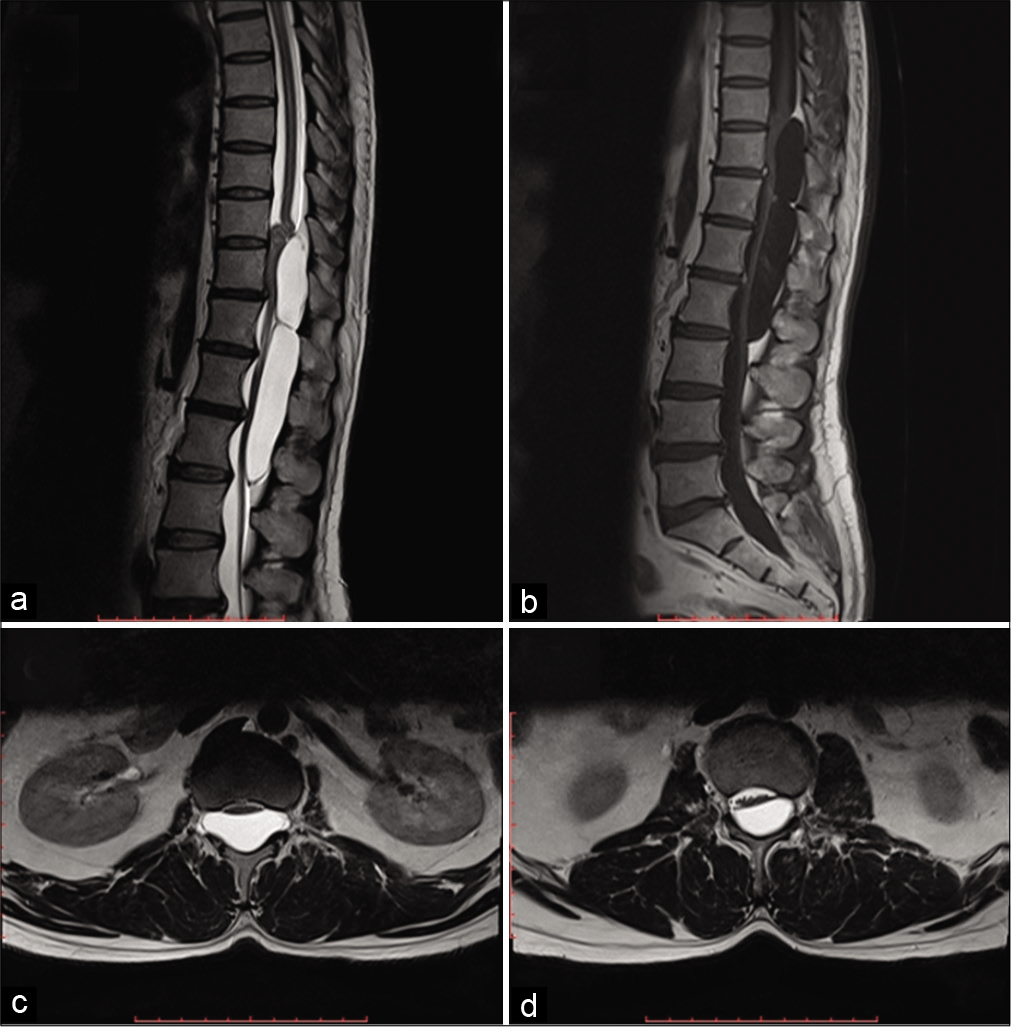
Figure 1:
(a) T2-W MRI sagittal images disclose a well-defined uniformly hyperintense (identical to CSF) cystic multiseptate lesion extending from the lower end of the T10 vertebral body to the lower end of L2 vertebral body. The bulk of the lesion is seen to create a mass effect and compression over the cauda equina. (b) T1-W MRI sagittal images after contrast injection reveal a well-defined uniform hypointense multiseptate lesion with no evidence of enhancement within the substance or peripheral wall of the lesion. (c and d) T2-W MRI axial images, at the level of T12 and L2, disclose well-defined uniform hyperintense lesion with compression and displacement of conus as well as caudal roots at respective levels. The lesion is seen to be extended into intervertebral foramen at the level of T12.
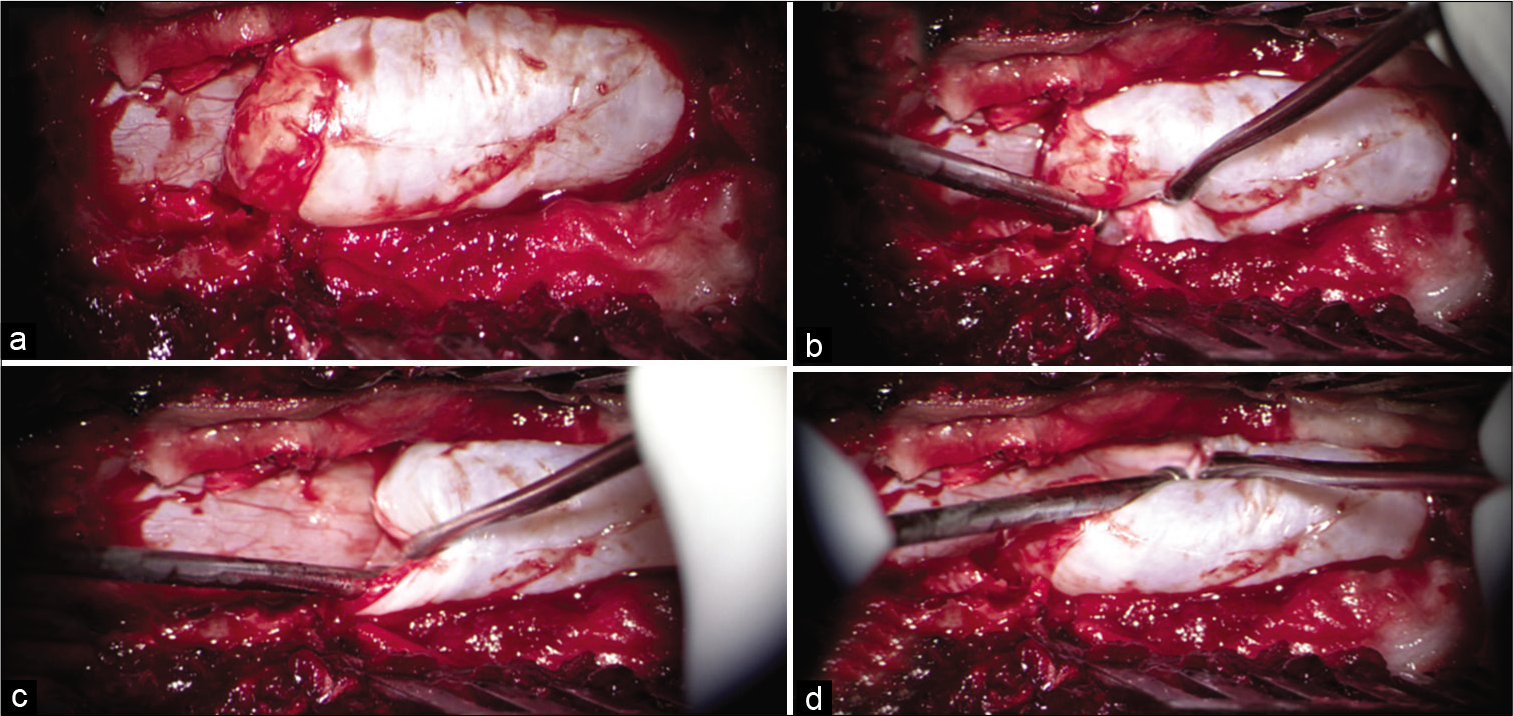
Figure 2:
(a) Intraoperative picture (under microscope) after T11-L2 laminectomy. Upper pole of large cystic lesion noted hanging over the underlying intact dura, while the inferior end is extending under L1 lamina which was preserved in tailored exposure. (b) Distended cyst wall is seen extending into the left neural foramina between T12 and L1 vertebral level. (c) Distinguished plane between cyst wall and native dura is clearly seen under the cranial pole of the cyst. (d) Distended cyst wall is seen extending into right neural foramina between T12 and L1 vertebral level.
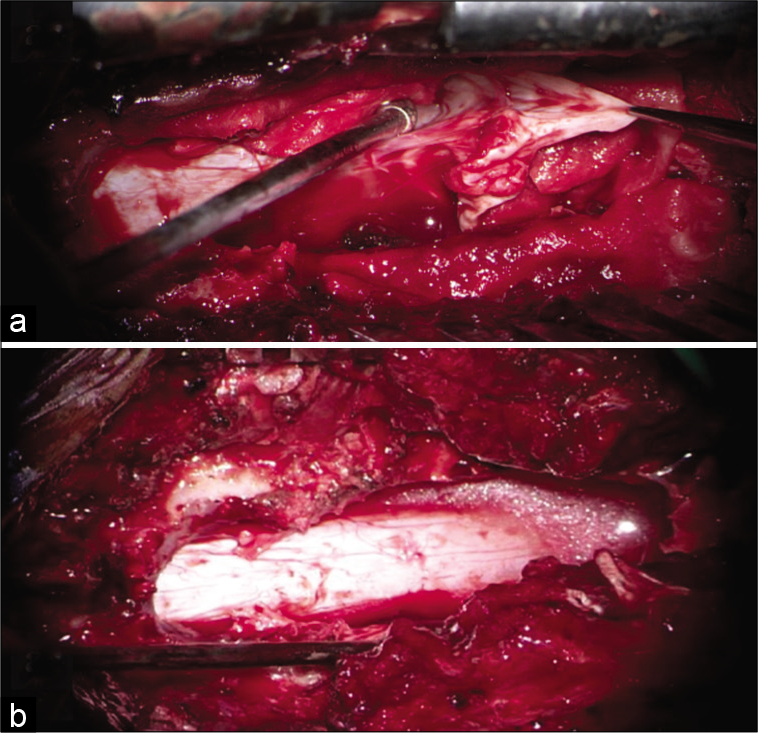
Figure 3:
(a) Intraoperative picture after fenestration and decompression of the cyst, the collapsed wall is seen extending into neural foramina between T12 and L1 vertebral level, where the dural defect was identified and repaired using 4–0 Prolene with muscle patch and fibrin glue. (b) After complete excision of cyst, dura is noted to be adequately decompressed (no evidence of CSF leak on Valsalva maneuver subsequently).
Pathology
The histopathological examination revealed collagenous tissue within the wall of the cyst with occasional cuboidal and flattened cells, suggestive of a meningothelial reaction.
Postoperative course
Immediately after surgery, the patient had dramatic relief in his symptoms. The follow-up MRI 3 months later confirmed complete excision of cyst and thecal sac decompression. By 9 postoperative months, the patient was completely asymptomatic.
DISCUSSION
Nabors three part classification of spinal arachnoid cysts
According to Nabors classification,[
Clinical manifestations of SEDAC are usually myelopathy, radiculopathy, or neurogenic bladder. SEDAC can either be congenital in etiology or sporadic. The former is caused by mutations in the FOXC2 gene and is inherited as a part of lymphedema–distichiasis syndrome.
Various surgical strategies have been described ranging from complete microsurgical resection to simple fenestration followed by closure of dural defect.[
Review of seventy prior SEDAC cases
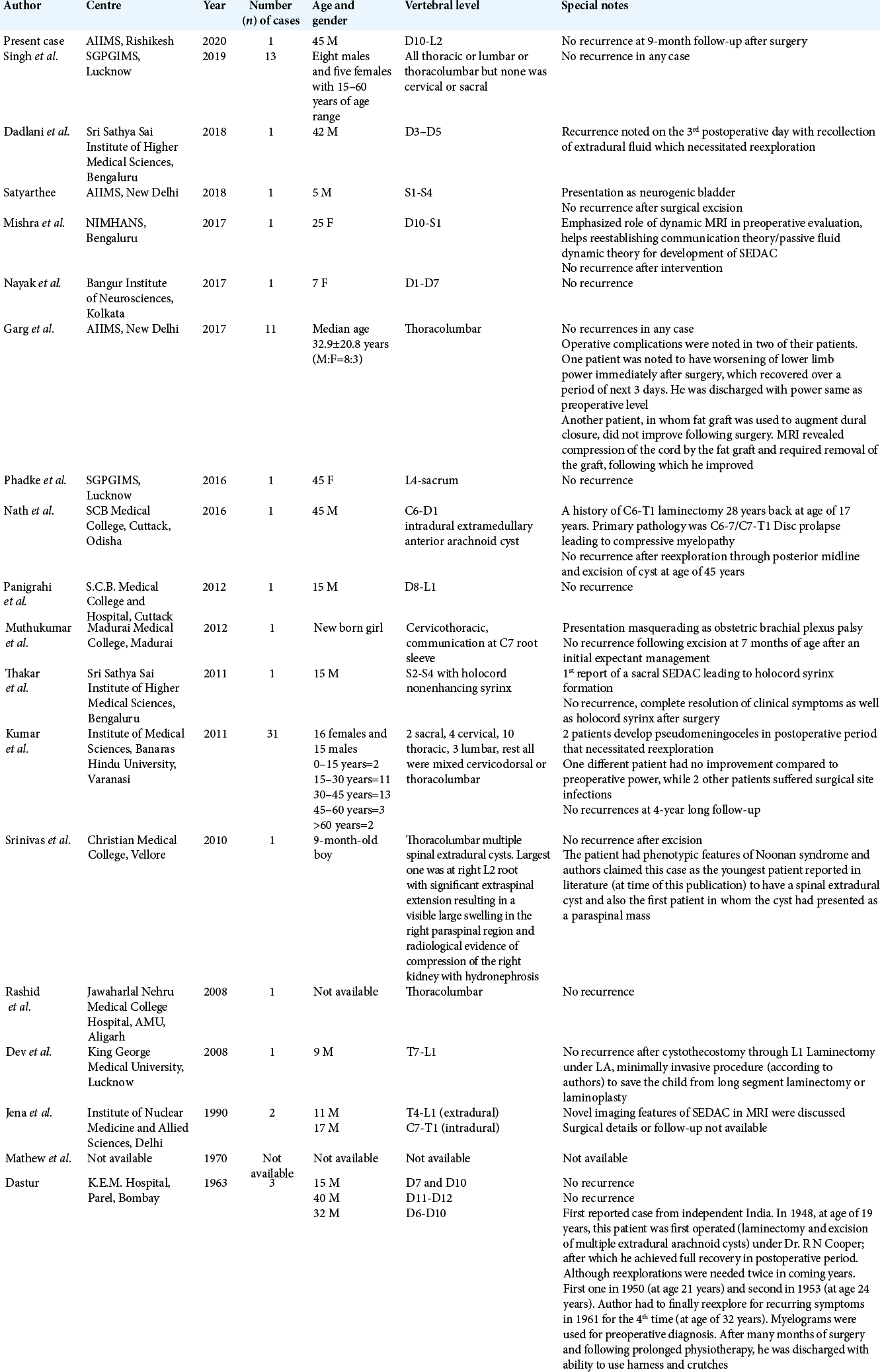
A total of seventy SEDAC cases have been reported, including 42 males and 28 females.[
CONCLUSION
Type 1 meningeal cysts can cause compressive myelopathy in the thoracolumbar region. Here, it was successfully managed with laminectomy, cyst excision, and direct repair of the dural defect.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Funao H, Nakamura M, Hosogane N, Watanabe K, Tsuji T, Ishii K. Surgical treatment of spinal extradural arachnoid cysts in the thoracolumbar spine. Neurosurgery. 2012. 71: 278-84
2. Furtado SV, Thakar S, Murthy GK, Dadlani R, Hegde AS. Management of complex giant spinal arachnoid cysts presenting with myelopathy. J Neurosurg Spine. 2011. 15: 107-12
3. Garg K, Borkar SA, Kale SS, Sharma BS. Spinal arachnoid cysts-our experience and review of literature. Br J Neurosurg. 2017. 31: 172-8
4. Kumar A, Sakia R, Singh K, Sharma V. Spinal arachnoid cyst. J Clin Neurosci. 2011. 18: 1189-92
5. Lee CH, Hyun SJ, Kim KJ, Jahng TA, Kim HJ. What is a reasonable surgical procedure for spinal extradural arachnoid cysts: Is cyst removal mandatory? Eight consecutive cases and a review of the literature. Acta Neurochir (Wien). 2012. 154: 1219-27
6. Nayak R, Chaudhuri A, Sadique S, Attry S. Multiple spinal extradural arachnoidal cysts: An uncommon cause of thoracic cord compression. Asian J Neurosurg. 2017. 12: 321-3
7. Netra R, Min L, Hui MS, Wang JC, Bin Y, Ming Z. Spinal extradural meningeal cysts: An MRI evaluation of a case series and literature review. J Spinal Disord Tech. 2011. 24: 132-6