- Department of Neurosurgery, Baylor College of Medicine,
- Department of Neurosurgery, University of Texas, MD Anderson Cancer Center,
- Department of Neurology, Baylor College of Medicine, Houston, Texas, United States.
Correspondence Address:
Sricharan Gopakumar
Department of Neurology, Baylor College of Medicine, Houston, Texas, United States.
DOI:10.25259/SNI_221_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sricharan Gopakumar, Marc Daou, Ron Gadot, Alexander E. Ropper, Jacob Mandel. Spinal meningioma in a patient with multiple sclerosis. 18-Jul-2020;11:196
How to cite this URL: Sricharan Gopakumar, Marc Daou, Ron Gadot, Alexander E. Ropper, Jacob Mandel. Spinal meningioma in a patient with multiple sclerosis. 18-Jul-2020;11:196. Available from: https://surgicalneurologyint.com/surgicalint-articles/10140/
Abstract
Background: Multiple sclerosis (MS) is the most common immune-mediated inflammatory demyelinating disease of the central nervous system. Multiple brain and spinal tumors have been linked to MS, but a causal relationship between the two has not been determined. Here, we report a case of spinal meningioma in a patient with MS and review literature discussing the possible connection between these two disease entities.
Case Description: A 58-year-old female with MS presented with a 1-year history of progressively worsening back pain in conjunction with worsening right upper and lower extremity weakness. The patient was diagnosed with MS 19 months prior and had multiple known demyelinating plaques in her cervical spine. New MRI revealed an intradural extramedullary thoracic tumor with characteristics consistent with meningioma. She underwent T6- T8 laminectomies for tumor resection and pathology confirmed the radiological diagnosis. At 3-month follow- up, the patient reported complete resolution of her back pain and persistence of weakness-related gait issues.
Conclusion: CNS neoplasms including meningioma should be considered in MS patients presenting with newly onset neurological symptoms not entirely consistent with demyelinating disease. Both disease processes should be addressed with appropriate long-term follow-up.
Keywords: Meningioma, Multiple sclerosis, Spine
INTRODUCTION
Multiple sclerosis (MS) is the most common immune-mediated inflammatory demyelinating disease of the central nervous system. Multiple brain and spinal tumors have been linked to MS, but a causal relationship between the two has not been determined. Here, we report a case of spinal meningioma in a patient with MS and review literature discussing the possible connection between these two disease entities.
CASE REPORT
A 58-year-old female with a medical history of hypertension, kidney stones, and multiple sclerosis (MS) presented for a second opinion with back pain and a 4-year history of right upper and lower extremity weakness that progressively worsened over the past year. She reported lower back pain and difficulty walking but denied radicular numbness, tingling, or any balance issues. The patient had previously been diagnosed with MS 19 months prior at an outside institution based on brain and cervical spine MR imaging and was being treated with Tecfidera and Amypra. On physical examination, the patient had a positive Babinski’s sign on the left. Decision was made to switch to ocrelizumab due to concern for primary progressive MS.
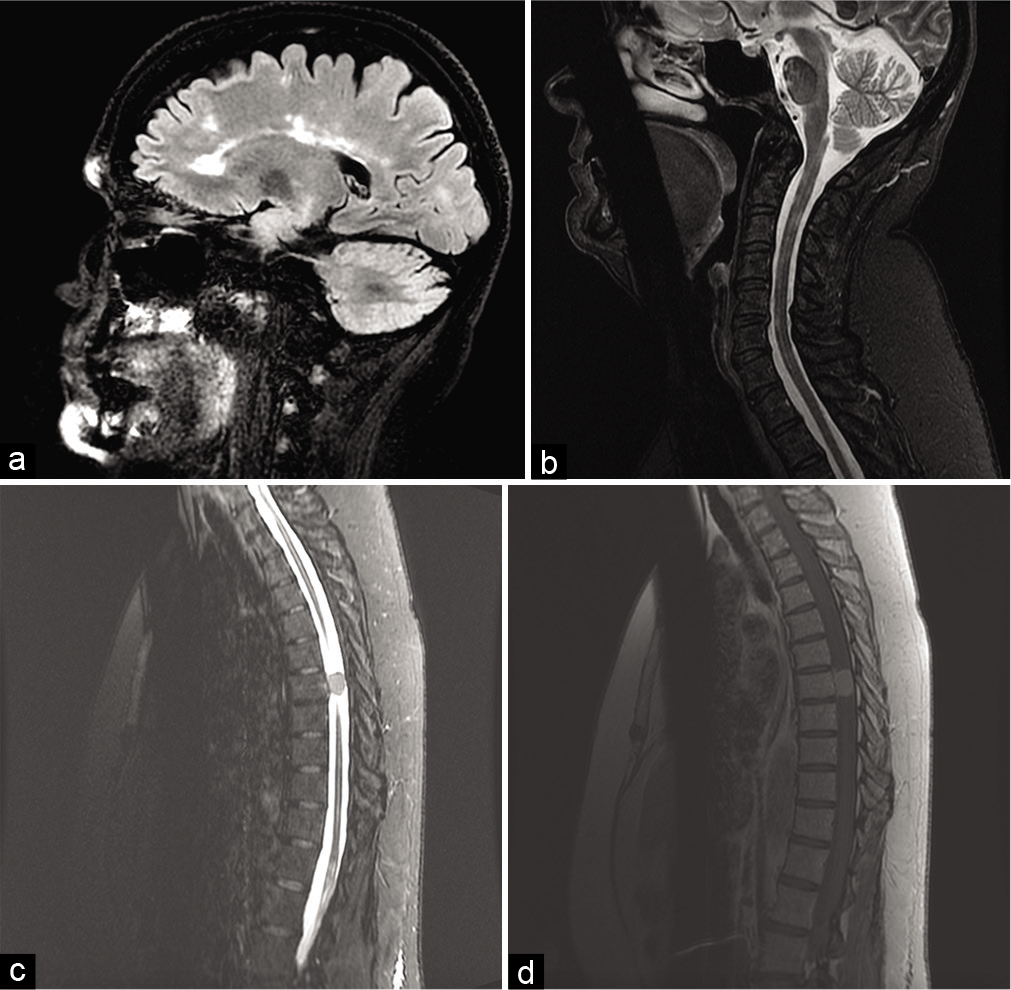
New MRI imaging of brain, cervical and thoracic spine was obtained [
Figure 1:
Magnetic resonance imaging (MRI) brain, cervical, and thoracic spine. (a) Sagittal fluid-attenuated inversion recovery brain MRI. More than 50 supra and infratentorial white matter lesions were observed with four new supratentorial and six new infratentorial lesions with no enhancing plaques to suggest acute demyelination. (b) Sagittal short-tau inversion-recovery (STIR) cervical MRI. Multiple demyelinating plaques were seen within the right anterior cord at the level of the dens and C2, the right posterolateral cord at C3 and C4, right lateral cord at C4 and C5, and the left posterior cord at levels C6–7. (c) Sagittal STIR thoracic MRI. Enhancing intradural extramedullary thoracic tumor occupies the left spinal canal at T7 with severe spinal cord stenosis and mass effect. (d) Sagittal T1 thoracic MRI with contrast. Dural tail extends superiorly.
Neurosurgery was consulted, and the patient underwent T6– T8 laminectomies for resection of the tumor. Pathology was consistent with a psammomatous meningioma, Grade 1. At 3-month follow-up after surgery, the patient reported a resolution of her back pain but continued to have issues with her gait.
DISCUSSION
MS patients have a decreased overall risk of cancer with the exception of CNS and genitourinary tumors, in which the risk is increased.[
Diagnostic neuroimaging is obtained in MS patients with new symptoms, and a CNS tumor should be ruled out before attributing symptoms to demyelinating disease.[
Uncommon neurological symptoms in an MS patient should raise the suspicion for non-MS-related lesions. The differential diagnoses of a pseudo-tumoral lesion include cancerous, infectious, metabolic, vascular, congenital, or idiopathic inflammatory etiologies.[
Due to its rarity, there are no trials for the treatment of meningioma associated with MS.[
CONCLUSION
If clinical symptoms and neuroimaging cannot rule out a CNS tumor and no improvement of symptoms is seen, definitive diagnosis should be attained from histological examination of either a stereotactic biopsy or surgically resected specimen. In the case of coexisting CNS tumor and MS, both conditions should be addressed individually, and the patient should continue to be followed for any MS relapses after tumor removal.
Declaration of patient consent
Patient’s consent not required as patient identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abrishamchi F, Khorvash F. Coexistence of multiple sclerosis and brain tumor: An uncommon diagnostic challenge. Adv Biomed Res. 2017. 6: 101-
2. Bahmanyar S, Montgomery SM, Hillert J, Ekbom A, Olsson T. Cancer risk among patients with multiple sclerosis and their parents. Neurology. 2009. 72: 1170-7
3. Barkhof F, Rocca M, Francis G, Van Waesberghe JH, Uitdehaag BM, Hommes OR. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon beta1a. Ann Neurol. 2003. 53: 718-24
4. Batay F, Al-Mefty O. Growth dynamics of meningiomas in patients with multiple sclerosis treated with interferon: Report of two cases. Acta Neurochir (Wien). 2002. 144: 365-8
5. Butteriss DJ, Ismail A, Ellison DW, Birchall D. Use of serial proton magnetic resonance spectroscopy to differentiate low grade glioma from tumefactive plaque in a patient with multiple sclerosis. Br J Radiol. 2003. 76: 662-5
6. Chamberlain MC, Glantz MJ. Interferon-alpha for recurrent World Health Organization grade 1 intracranial meningiomas. Cancer. 2008. 113: 2146-51
7. Drevelegas A, Xinou E, Karacostas D, Parissis D, Karkavelas G, Milonas I. Meningioma growth and interferon beta-1b treated multiple sclerosis: Coincidence or relationship?. Neuroradiology. 2005. 47: 516-9
8. Hemminki K, Liu X, Försti A, Ji J, Sundquist J, Sundquist K. Subsequent brain tumors in patients with autoimmune disease. Neuro Oncol. 2013. 15: 1142-50
9. Malhotra HS, Jain KK, Agarwal A, Singh MK, Yadav SK, Husain M. Characterization of tumefactive demyelinating lesions using MR imaging and in vivo proton MR spectroscopy. Mult Scler. 2009. 15: 193-203
10. Plantone D, Renna R, Sbardella E, Koudriavtseva T. Concurrence of multiple sclerosis and brain tumors. Front Neurol. 2015. 6: 40-