- Department of Neurosurgery, Brigham and Women’s Hospital, Boston, Massachusetts, United States.
- Department of Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, United States.
- Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, United States.
Correspondence Address:
Melissa Ming Jie Chua
Department of Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, United States.
DOI:10.25259/SNI_446_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Melissa Ming Jie Chua1, Alvin S. Das2, Julie Aurore Losman3, Nirav J. Patel1, Saef Izzy2. Spontaneous hemorrhage after external ventricular drain placement in the setting of low factor VII secondary to liver cirrhosis. 25-Nov-2020;11:403
How to cite this URL: Melissa Ming Jie Chua1, Alvin S. Das2, Julie Aurore Losman3, Nirav J. Patel1, Saef Izzy2. Spontaneous hemorrhage after external ventricular drain placement in the setting of low factor VII secondary to liver cirrhosis. 25-Nov-2020;11:403. Available from: https://surgicalneurologyint.com/surgicalint-articles/10408/
Abstract
Background: Alterations in normal coagulation and hemostasis are critical issues that require special attention in the neurosurgical patient. These disorders pose unique challenges in the management of these patients who often have concurrent acute ischemic and hemorrhagic injuries. Although neurosurgical intervention in such cases may be unavoidable and potentially life-saving, these patients should be closely observed after instrumentation.
Case Description: A 57-year-old male with liver cirrhosis secondary to amyloid light-chain amyloidosis was admitted to the intensive care unit for the management of delayed hydrocephalus. An external ventricular drain (EVD) was placed for the treatment and monitoring of hydrocephalus. Five days after EVD placement, a head computed tomography scan revealed a tract hemorrhage. However, on repeated imaging, the size of the hemorrhage continued to increase despite aggressive blood pressure control and several doses of phytonadione. Extensive coagulopathy workup was remarkable for low factor VII levels. In that setting, recombinant activated factor VII was administered to normalize factor VII levels, and the tract hemorrhage stabilized.
Conclusion: To the best of our knowledge, this is the first case of spontaneous hemorrhage after EVD placement in the setting of liver cirrhosis-associated factor VII deficiency. Our case highlights the importance of identifying coagulation disorders in neurosurgical patients at high risk for coagulopathy and closely monitoring them postoperatively.
Keywords: Coagulopathy, Factor VII, Liver cirrhosis
INTRODUCTION
Hemostasis is particularly important in neurosurgical patients as minor abnormalities can pose significant bleeding risks leading to worsened morbidity and mortality. As such, coagulopathy disorders should be diagnosed and managed appropriately, especially after instrumentation. Common causes of acquired coagulopathies in neurosurgical patients include antithrombotic use, thrombocytopenia, sepsis, disseminated intravascular coagulation, uremia, and liver disease.[
CASE DESCRIPTION
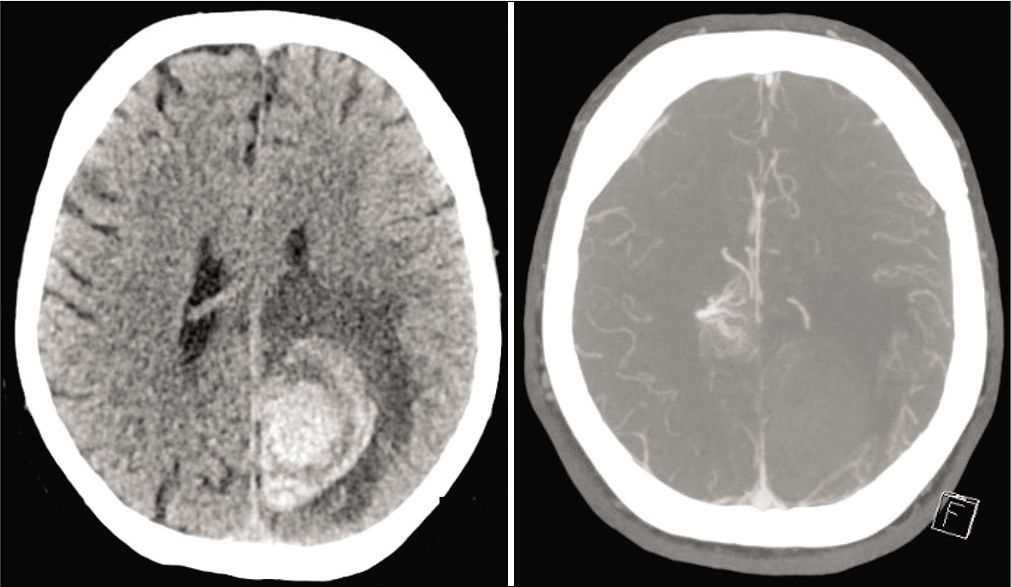
A 57-year-old Caucasian male with atrial fibrillation (not on anticoagulation), hypertension, monoclonal gammopathy of undetermined significance, and AL amyloidosis with known liver involvement leading to cirrhosis initially presented to his local emergency department with right-sided weakness and headache. His blood pressure on presentation was 164/78 mmHg, and he was found to have a left parietal intraparenchymal hemorrhage and an incidental right parietal arteriovenous malformation [
He was readmitted 1 month later with severe headaches and worsening right-sided weakness (3-/5 strength) with a blood pressure of 148/73 mmHg. A head computed tomography (CT) revealed an increase in size of the left parietal hemorrhage. At that time, he also experienced epistaxis as well as catheter site bleeding that resolved spontaneously. Laboratory workup showed a stable platelet count of 127 × 109/L, normal factor X activity (338%), normal von Willebrand factor activity (736%), and a mildly elevated international normalized ratio (INR) of 1.4 for which he was given intravenous (IV) Vitamin K (phytonadione). Interval imaging demonstrated stability of the intraparenchymal hemorrhage; however, due to concern for seizures and altered mental status, he had a prolonged hospitalization on the neurology service. He was subsequently discharged back to rehabilitation with an antiepileptic drug regimen of levetiracetam (2000 mg twice daily), clobazam (20 mg twice daily), and lacosamide (50 mg daily).
The patient was readmitted 2 months later to our institution for worsening encephalopathy. A head CT showed a mild increase in ventricular size in addition to the resolving left parietal intraparenchymal hemorrhage. An electroencephalogram and extensive metabolic and infectious workup were unremarkable. Given the possibility of delayed hydrocephalus as explanation for his worsening encephalopathy, the patient was trialed on cerebrospinal fluid diversion with an EVD [
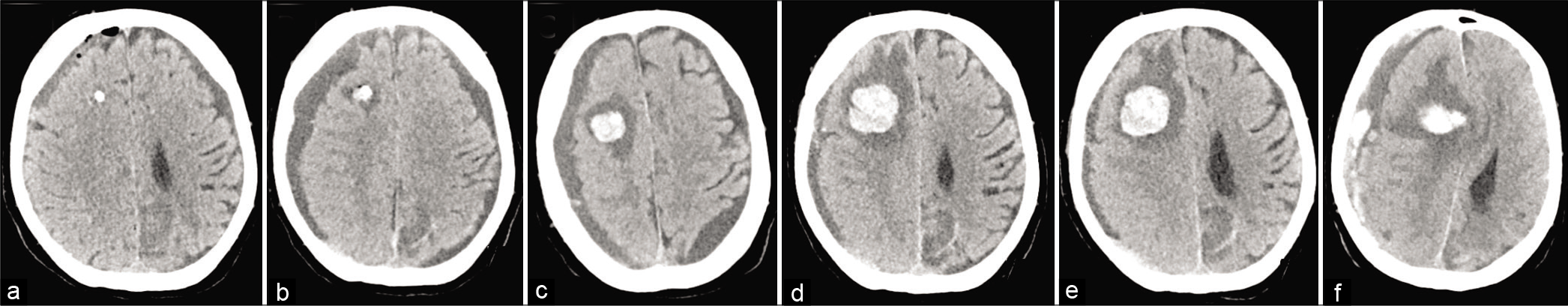
Figure 2:
Noncontrast head computed tomography scans, axial views. (a) Immediately post-external ventricular drain (EVD) placement. (b) Spontaneous tract hemorrhage 5 days post-EVD placement. Interval enlargement of the EVD tract hemorrhage with 6 days (c) and 8 days. (d) Post-EVD placement shown here. (e) Recombinant factor VII was given on post-EVD placement day 8 with stabilization of the tract hemorrhage on day 9. (f) Acute development of the right-sided subdural hemorrhage 10-day post-EVD placement.
DISCUSSION
We report a rare case of worsening hemorrhage almost 1 week after EVD placement in the setting of an acquired VII deficiency from liver cirrhosis secondary to AL amyloidosis. Liver cirrhosis is a known cause of coagulopathy which can complicate the postoperative management of a neurosurgical patient. Studies have shown up to 2.4% incidence of major bleeding after invasive procedures in cirrhotic patients.[
Liver disease can often lead to low platelet counts and platelet dysfunction.[
rFVIIa is only approved for replacement therapy in congenital factor VII deficiency where the recommended dose is 15–30 mcg/kg every 4–6 h until hemostasis is attained.[
Randomized clinical trials using rFVIIa in patients presenting with intracranial hemorrhage have yielded mixed results. In one study of anticoagulant-related intracranial hemorrhage, rFVIIa was effective in normalizing INR without producing any thromboembolic complications.[
CONCLUSION
Our case highlights the importance of identifying coagulation disorders in neurosurgical patients at high risk for coagulopathy and closely monitoring them postoperatively. Particularly in cases where coagulopathies are unable to be reversed, there should be a low threshold to check coagulation factor levels including factor VII. In cases where correction of low factor VII levels do not achieve adequate hemostasis, aminocaproic acid should be considered as it was shown to be effective in our patient. To the best of our knowledge, this is the first case of spontaneous hemorrhage after EVD placement in the setting of liver cirrhosis-associated factor VII deficiency.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aldrich SM, Regal RE. Routine use of vitamin k in the treatment of cirrhosis-related coagulopathy: Is it A-O-K?. Maybe not we say. P T. 2019. 44: 131-6
2. Bui JD, Despotis GD, Trulock EP, Patterson GA, Goodnough LT. Fatal thrombosis after administration of activated prothrombin complex concentrates in a patient supported by extracorporeal membrane oxygenation who had received activated recombinant factor VII. J Thorac Cardiovasc Surg. 2002. 124: 852-4
3. DeAngelis GA, Khot R, Haskal ZJ, Maitland HS, Northup PG, Shah NL. Bleeding risk and management in interventional procedures in chronic liver disease. J Vasc Interv Radiol. 2016. 27: 1665-74
4. Drolz A, Ferlitsch A, Fuhrmann V. Management of coagulopathy during bleeding and invasive procedures in patients with liver failure. Visc Med. 2018. 34: 254-8
5. Dutta TK, Verma SP. Rational use of recombinant factor VIIa in clinical practice. Indian J Hematol Blood Transfus. 2014. 30: 85-90
6. Gladstone DJ, Aviv RI, Demchuk AM, Hill MD, Thorpe KE, Khoury JC. Effect of recombinant activated coagulation factor VII on hemorrhage expansion among patients with spot sign-positive acute intracerebral hemorrhage: The SPOTLIGHT and STOP-IT randomized clinical trials. JAMA Neurol. 2019. 76: 1493-501
7. Goodnough LT, Shander AS. Recombinant factor VIIa: Safety and efficacy. Curr Opin Hematol. 2007. 14: 504-9
8. Kaseer H, Sanghavi D.editors. Aminocaproic acid. Stat Pearls. Treasure Island, FL: Stat Pearls Publishing; 2020. p.
9. Li J, Han B, Li H, Deng H, Méndez-Sánchez N, Guo X. Association of coagulopathy with the risk of bleeding after invasive procedures in liver cirrhosis. Saudi J Gastroenterol. 2018. 24: 220-7
10. Lisman T, Caldwell SH, Burroughs AK, Northup PG, Senzolo M, Stravitz RT. Hemostasis and thrombosis in patients with liver disease: The ups and downs. J Hepatol. 2010. 53: 362-71
11. Lisman T, Porte RJ. Value of preoperative hemostasis testing in patients with liver disease for perioperative hemostatic management. Anesthesiology. 2017. 126: 338-44
12. Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008. 358: 2127-37
13. Medow JE, Dierks MR, Williams E, Zacko JC. The emergent reversal of coagulopathies encountered in neurosurgery and neurology: A technical note. Clin Med Res. 2015. 13: 20-31
14. Muciño-Bermejo J, Carrillo-Esper R, Uribe M, MéndezSánchez N. Coagulation abnormalities in the cirrhotic patient. Ann Hepatol. 2013. 12: 713-24
15. Nadim MK, Durand F, Kellum JA, Levitsky J, O’Leary JG, Karvellas CJ. Management of the critically ill patient with cirrhosis: A multidisciplinary perspective. J Hepatol. 2016. 64: 717-35
16. Napolitano G, Iacobellis A, Merla A, Niro G, Valvano MR, Terracciano F. Bleeding after invasive procedures is rare and unpredicted by platelet counts in cirrhotic patients with thrombocytopenia. Eur J Intern Med. 2017. 38: 79-82
17. Nguyen AL, Kamal M, Raghavan R, Nagaraj G. Acquired factor VII deficiency causing severe bleeding disorder secondary to AL amyloidosis of the liver. Hematol Rep. 2018. 10: 7235
18. O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006. 295: 293-8
19. Park P, Fewel ME, Garton HJ, Thompson BG, Hoff JT. Recombinant activated factor VII for the rapid correction of coagulopathy in nonhemophilic neurosurgical patients. Neurosurgery. 2003. 53: 34-8
20. Peerlinck K, Vermylen J. Acute myocardial infarction following administration of recombinant activated factor VII (Novo seven) in a patient with haemophilia A and inhibitor. Thromb Haemost. 1999. 82: 1775-6
21. Rivosecchi RM, Kane-Gill SL, Garavaglia J, MacLasco A, Johnson H. The effectiveness of intravenous Vitamin K in correcting cirrhosis-associated coagulopathy. Int J Pharm Pract. 2017. 25: 463-5
22. Rosenfeld SB, Watkinson KK, Thompson BH, Macfarlane DE, Lentz SR. Pulmonary embolism after sequential use of recombinant factor VIIa and activated prothrombin complex concentrate in a factor VIII inhibitor patient. Thromb Haemost. 2002. 87: 925-6
23. Saja MF, Abdo AA, Sanai FM, Shaikh SA, Gader AG. The coagulopathy of liver disease: Does Vitamin K help?. Blood Coagul Fibrinolysis. 2013. 24: 10-7
24. Saner FH, Kirchner C. Monitoring and treatment of coagulation disorders in end-stage liver disease. Visc Med. 2016. 32: 241-8
25. Schepis F, Turco L, Bianchini M, Villa E. Prevention and management of bleeding risk related to invasive procedures in cirrhosis. Semin Liver Dis. 2018. 38: 215-29
26. Shah A, Amarapurkar D, Dharod M, Chandnani M, Baijal R, Kumar P. Coagulopathy in cirrhosis: A prospective study to correlate conventional tests of coagulation and bleeding following invasive procedures in cirrhotics. Indian J Gastroenterol. 2015. 34: 359-64
27. Townsend JC, Heard R, Powers ER, Reuben A. Usefulness of international normalized ratio to predict bleeding complications in patients with end-stage liver disease who undergo cardiac catheterization. Am J Cardiol. 2012. 110: 1062-5
28. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011. 365: 147-56
29. Vanderwerf JD, Kumar MA. Management of neurologic complications of coagulopathies. Handb Clin Neurol. 2017. 141: 743-64
30. Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: Randomized clinical trial. Transplant Proc. 2010. 42: 2590-3
31. Yampolsky N, Stofko D, Veznedaroglu E, Liebman K, Binning MJ. Recombinant factor VIIa use in patients presenting with intracranial hemorrhage. Springerplus. 2014. 3: 471