- Department of Neurosurgery, University of Messina, Messina, Italy
- Department of Neurosurgery, University of Palermo, Palermo, Italy
- Department of Neuroradiology, University of Messina, Messina, Italy
Correspondence Address:
Concetta Alafaci
Department of Neurosurgery, University of Messina, Messina, Italy
DOI:10.4103/2152-7806.166776
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alafaci C, Grasso G, Granata F, Marino D, Salpietro FM, Tomasello F. Spontaneous Meckel's cave hematoma: A rare cause of trigeminal neuralgia. Surg Neurol Int 07-Oct-2015;6:
How to cite this URL: Alafaci C, Grasso G, Granata F, Marino D, Salpietro FM, Tomasello F. Spontaneous Meckel's cave hematoma: A rare cause of trigeminal neuralgia. Surg Neurol Int 07-Oct-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/spontaneous-meckels-cave-hematoma-a-rare-cause-of-trigeminal/
Abstract
Background:The most common etiology of classic trigeminal neuralgia (TN) is vascular compression. However, other causes must be considered. Among these, spontaneous hematoma of the Meckel's cave (MC) causing symptomatic TN is very rare.
Case Description:We present the case of a 60-year-old woman with a 2-month history of left TN and diplopia. Neuroradiological examinations revealed a well-defined hematoma in the left MC. The patient underwent surgical decompression with a progressive neurological improvement.
Conclusion:Despite the number of lesions potentially affecting the MC, spontaneous hemorrhage is rare but should be taken into account in the differential diagnosis.
Keywords: Intracranial hemorrhage, Meckel's cave, trigeminal neuralgia
INTRODUCTION
Typical trigeminal neuralgia (TN) is characterized by short periods of excruciating pain in a particular area of the face. The pain is paroxysmal and recurrent, lasting from a fraction of a second to few minutes. This occurrence has an estimated annual incidence of about 4.5/100,000 in the general population for both sexes.[
TN in its etiology is divided into a classic form, caused by vascular compression of the trigeminal nerve root, and symptomatic one, caused by other factors as tumors, vascular disorders, and demyelination in multiple sclerosis.[
CASE REPORT
Clinical presentation
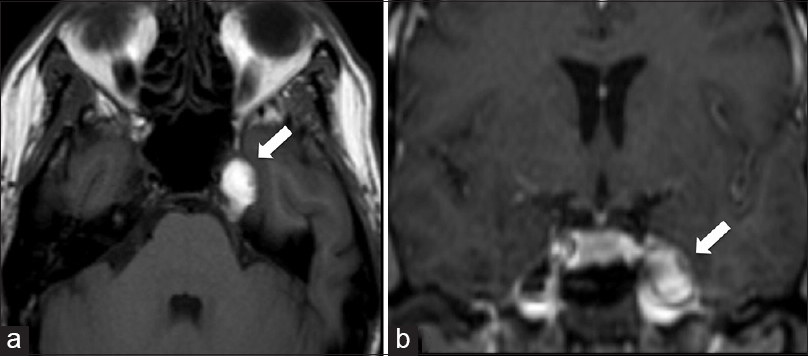
A 60-year-old woman was admitted with a 2-month history of the left mild TN and numbness on the left face. A double vision on the right lateral gaze was also reported. The neurological examination revealed a superficial hypoesthesia on the second branch of the left trigeminal nerve and a VI cranial nerve palsy. The patient's medical history was unremarkable except for slight systemic hypertension and unspecified surgery facelift occurred 2 years before admission. Magnetic resonance imaging (MRI) showed a round-shaped lesion, hyperintense in T1-weighted sequences, occupying the left MC [
Treatment
The patient underwent surgical treatment. A left mini-pterional approach was performed, and a lumbar drainage was placed at the beginning of the procedure, to allow an optimal brain parenchyma relaxation. The dura of the MC was opened, and blood clots were removed achieving optimal decompression. Specimens were sent for histological examination.
The postoperative course was uneventful. The TN disappeared and the VI cranial nerve palsy gradually recovered. The sensory disturbances on the left part of the face improved, being scarcely observed at the 8-month follow-up. Histological examination did not show any pathological lesion but only erythrocytes and rare polymorphonuclear granulocytes, confirming the diagnosis of spontaneous hemorrhage.
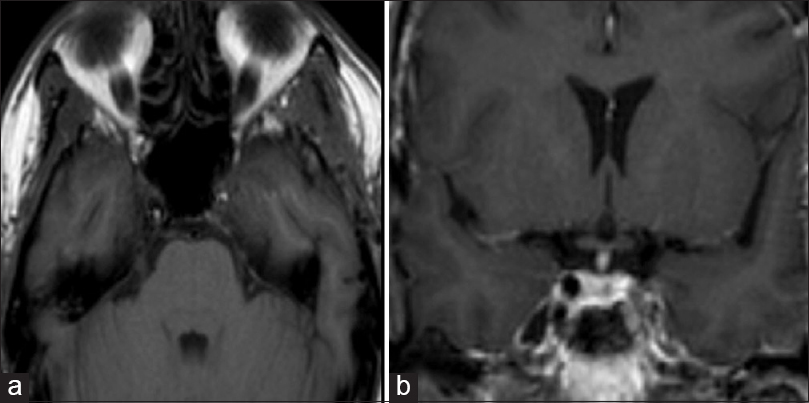
Brain MRI, performed 8 months later, showed the complete disappearance of the hematoma [
DISCUSSION
Although there is no proven etiology of TN, some autopsy results in patients with TN and multiple sclerosis indicate that demyelination within the descending tract of the trigeminal nerve or within the nerve itself can be correlated with the disorder.[
Besides vascular compression, other structural lesions such as tumors,[
The mechanisms for selective bleeding into the MC are unknown. It could be speculated that small tumors arising into the MC can bleed and destroy themselves by the acute hemorrhage so as histology cannot detect pathologic features. Beside this suggestive hypothesis, systemic hypertension should be considered as a possible cause. Nontraumatic intracerebral hemorrhage the most commonly results from hypertensive damage to blood vessel walls, but it also may be due to autoregulatory dysfunction with excessive cerebral blood flow. It is well known that chronic hypertension produces a small vessel vasculopathy. Such a vasculopathy is mainly characterized by lipohyalinosis, fibrinoid necrosis, and development of Charcot-Bouchard aneurysms, affecting penetrating arteries throughout the brain.[
Given the peculiarity of our findings, the reported case points out the importance of the knowledge of such a pathological condition when dealing with patients affected by TN. Accordingly, Meckel's cave hematoma should be considered, and a differential diagnosis should be made with hemorrhagic tumors such as trigeminal schwannomas or arteriovenous malformations in the MC.
MC pathology includes a large variety of tumors that are, in order of decreasing frequency, schwannomas, meningiomas, epidermoids, dermoids, hemangiopericytomas, metastases, lipomas, xanthomas, and lymphomas. The largest single-institution series so far reported by Muto et al. in 2010 includes 37 cases, most of them being schwannomas.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alafaci C, Caffo M, Barresi V, Cutugno M, Pino MA, Granata F. Large trigeminal schwannoma of the infratemporal fossa: Evaluation of neoangiogenesis in this rare neoplasm. Head Neck. 2013. 35: E272-6
2. Alafaci C, Salpietro FM, Puglisi E, Tripodo E, Matalone D, Di Pietro G. Trigeminal pain caused by a cerebellopontine-angle lipoma. Case report and review of the literature. J Neurosurg Sci. 2001. 45: 110-3
3. Cirak B, Kiymaz N, Arslanoglu A. Trigeminal neuralgia caused by intracranial epidermoid tumor: Report of a case and review of the different therapeutic modalities. Pain Physician. 2004. 7: 129-32
4. Douvrin F, Ménif E, Callonnec F, Thiébot J. What is your diagnosis. Trigeminal neuralgia caused by a hematoma in Meckel's cavity?. J Neuroradiol. 1999. 26: 213-4
5. Evers S. The new IHS classification. Background and structure. Schmerz. 2004. 18: 351-6
6. Farri A, Enrico A, Farri F. Headaches of otolaryngological interest: Current status while awaiting revision of classification. Practical considerations and expectations. Acta Otorhinolaryngol Ital. 2012. 32: 77-86
7. Gardner WJ, Miklos MV. Response of trigeminal neuralgia to decompression of sensory root; discussion of cause of trigeminal neuralgia. J Am Med Assoc. 1959. 170: 1773-6
8. Grasso G, Passalacqua M, Giambartino F, Cacciola F, Caruso G, Tomasello F. Typical trigeminal neuralgia by an atypical compression: Case report and review of the literature. Turk Neurosurg. 2014. 24: 82-5
9. Jannetta PJ. Neurovascular compression in cranial nerve and systemic disease. Ann Surg. 1980. 192: 518-25
10. Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: Similarities and differences, Rochester, Minnesota, 1945-1984. Neuroepidemiology. 1991. 10: 276-81
11. Levitt MR, Ramanathan D, Vaidya SS, Hallam DK, Ghodke BV. Endovascular palliation of AVM-associated intractable trigeminal neuralgia via embolization of the artery of the foramen rotundum. Pain Med. 2011. 12: 1824-30
12. Maggioni F, Bellamio M, Margoni M, Zanchin G, Manara R. Hematoma of Meckel's cave: A rare possible symptomatic trigeminal neuralgia. J Neurol. 2012. 259: 1481-2
13. Muto J, Kawase T, Yoshida K. Meckel's cave tumors: Relation to the meninges and minimally invasive approaches for surgery: Anatomic and clinical studies. Neurosurgery. 2010. 67: 291-8
14. Wang C, Wang YN, Sun K, Yin J, Xiao HS, Zhong J. A successful treatment of coexistent trigeminal neuralgia and ipsilateral superior cerebellar artery aneurysm. J Craniofac Surg. 2015. 26: 1270-2
15. Woo D, Haverbusch M, Sekar P, Kissela B, Khoury J, Schneider A. Effect of untreated hypertension on hemorrhagic stroke. Stroke. 2004. 35: 1703-8