- Department of Neurosurgery, Tsukuba Memorial Hospital, Tsukuba, Japan.
- Department of Neurosurgery, University of Tsukuba, Tsukuba, Japan.
Correspondence Address:
Eiichi Ishikawa, Department of Neurosurgery, University of Tsukuba, Tsukuba, Japan.
DOI:10.25259/SNI_429_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Maya Takada1, Kiyoyuki Yanaka1, Kazuhiro Nakamura1, Ken Akimoto1, Hayato Takeda1, Eiichi Ishikawa2. Spontaneous regression of a posterior fossa meningioma: A case report. 05-Aug-2022;13:334
How to cite this URL: Maya Takada1, Kiyoyuki Yanaka1, Kazuhiro Nakamura1, Ken Akimoto1, Hayato Takeda1, Eiichi Ishikawa2. Spontaneous regression of a posterior fossa meningioma: A case report. 05-Aug-2022;13:334. Available from: https://surgicalneurologyint.com/surgicalint-articles/11773/
Abstract
Background: Since most incidentally discovered meningiomas grow or remain unchanged, spontaneous regression is extremely rare. Here, we report a case of posterior fossa meningioma showing spontaneous regression.
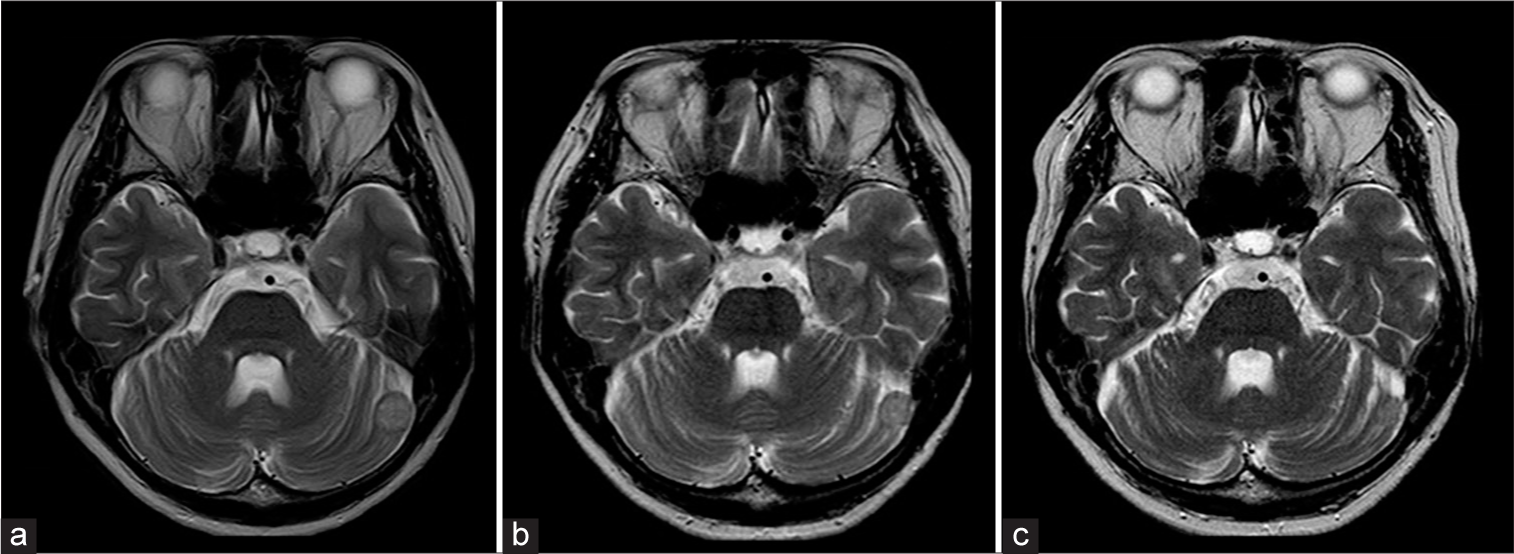
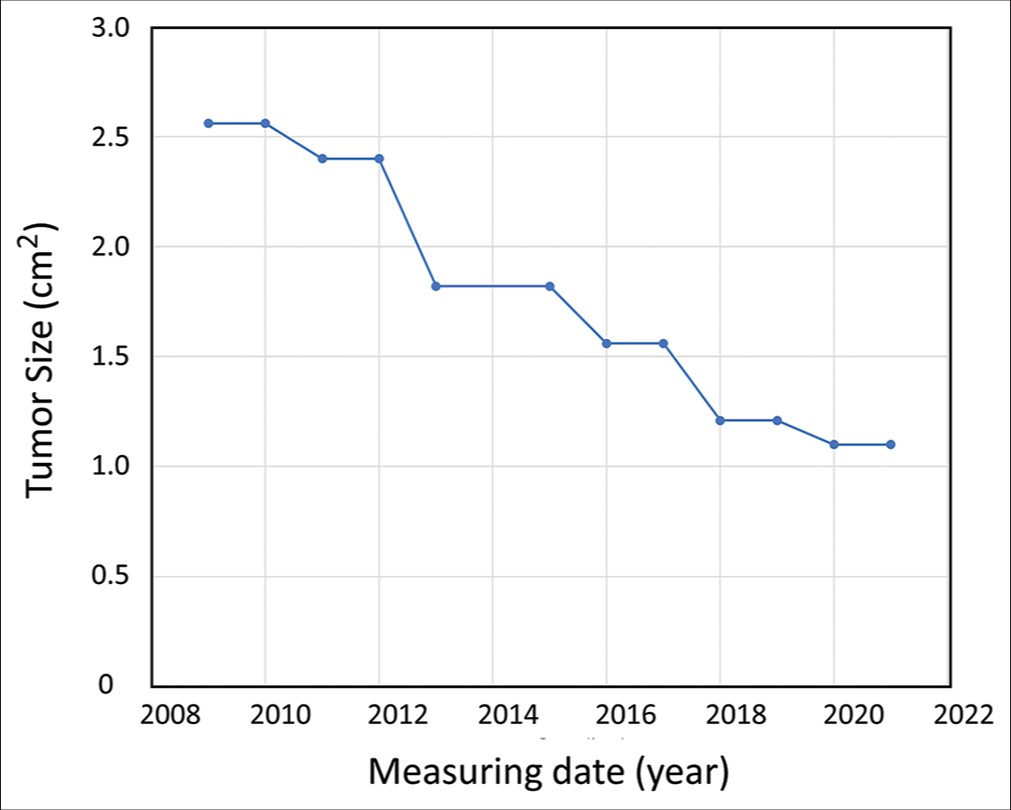
Case Description: A 55-year-old female was referred to our hospital because she was diagnosed with a left posterior fossa meningioma (diameter: 1.6 cm) during a brain check-up. The patient was followed up on periodic magnetic resonance imaging studies. Tumor size remained almost unchanged for 2 years but then began to regress. Twelve years after the initial examination, the tumor diameter idiopathically decreased from 1.6 cm to 1.1 cm while the tumor volume decreased from 2.3 cm3 to 0.5 cm3 (about 1/4th the original size). Postmenopausal hormonal imbalances may have been associated with the observed spontaneous regression.
Conclusion: Understanding the natural history of meningiomas is essential for a better selection of treatment approaches or appropriate follow-up. This case may provide new insights into the progression of meningiomas.
Keywords: Asymptomatic, Meningioma, Posterior fossa, Spontaneous regression
INTRODUCTION
Meningiomas, reported to account for 13–26% of all primary intracranial tumors,[
CASE PRESENTATION
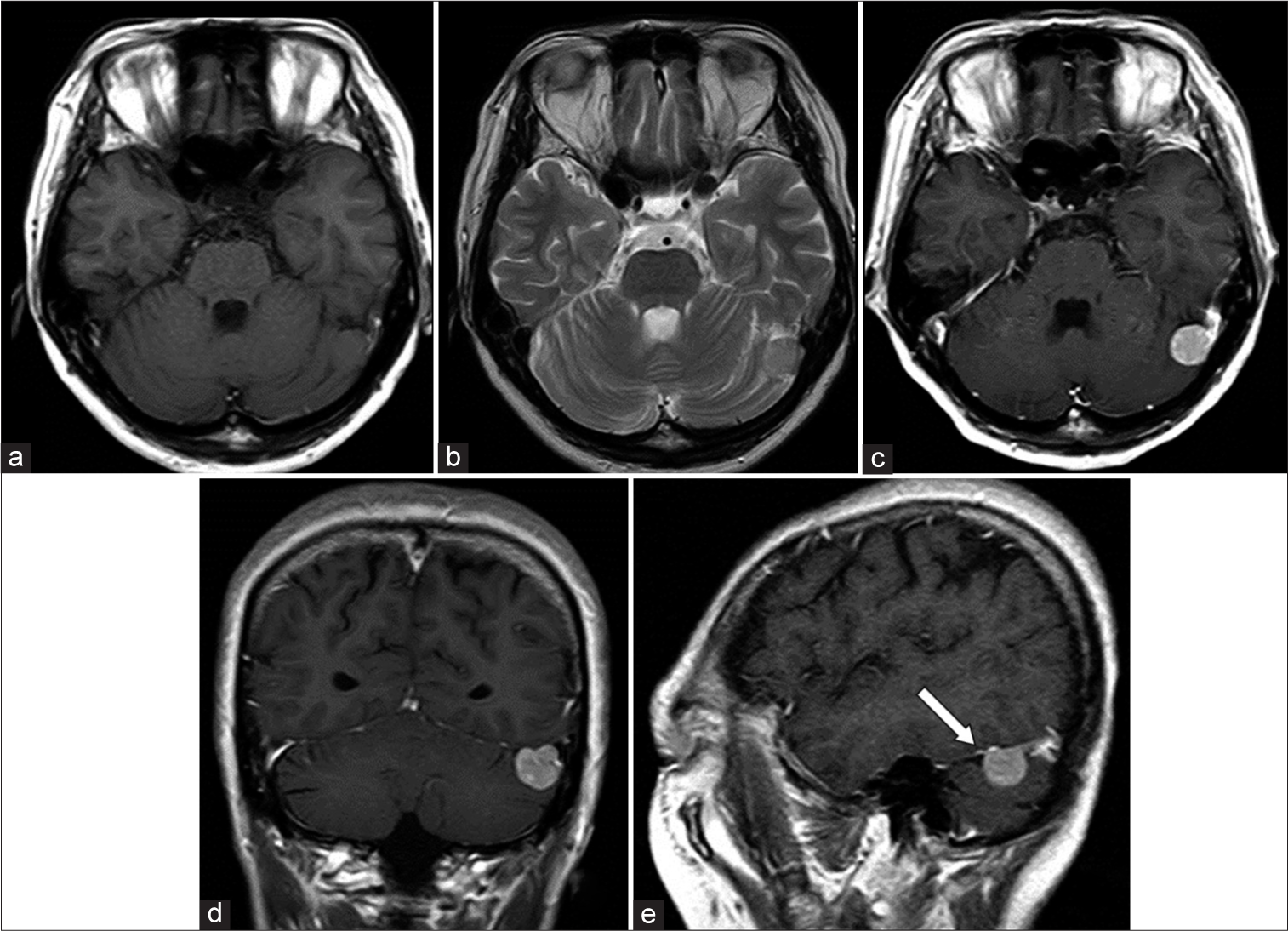
A 55-year-old woman diagnosed with a brain tumor during a brain check-up was referred to our hospital for further examination in April 2009. On admission, she had no neurological deficits, with a medical history of uterine myoma and no risk factors for atherosclerosis. Menopause had occurred in her early 50s. MR imaging showed a round-shaped mass near the left transverse-sigmoid junction protruding into the posterior fossa (1.6 cm × 1.6 cm × 1.7 cm). The tumor appeared as an isointense mass on T1-weighted imaging (WI) and slightly hyperintense on T2WI with enhancement after gadolinium administration [
Due to the increased size of the uterine myoma, the patient underwent hysterectomy and bilateral oophorectomy in 2019 (10 years after discovering the brain tumor) but the brain tumor remains asymptomatic.
DISCUSSION
Asymptomatic tumor discovery has increased with the spread of diagnostic imaging and the advent of an aging society. Therefore, it is essential to know the progressive nature of a disease to develop an appropriate treatment and follow-up policy since tumors generally grow over time but some may exhibit spontaneous regression. Almost all varieties of malignant tumors can spontaneously regress,[
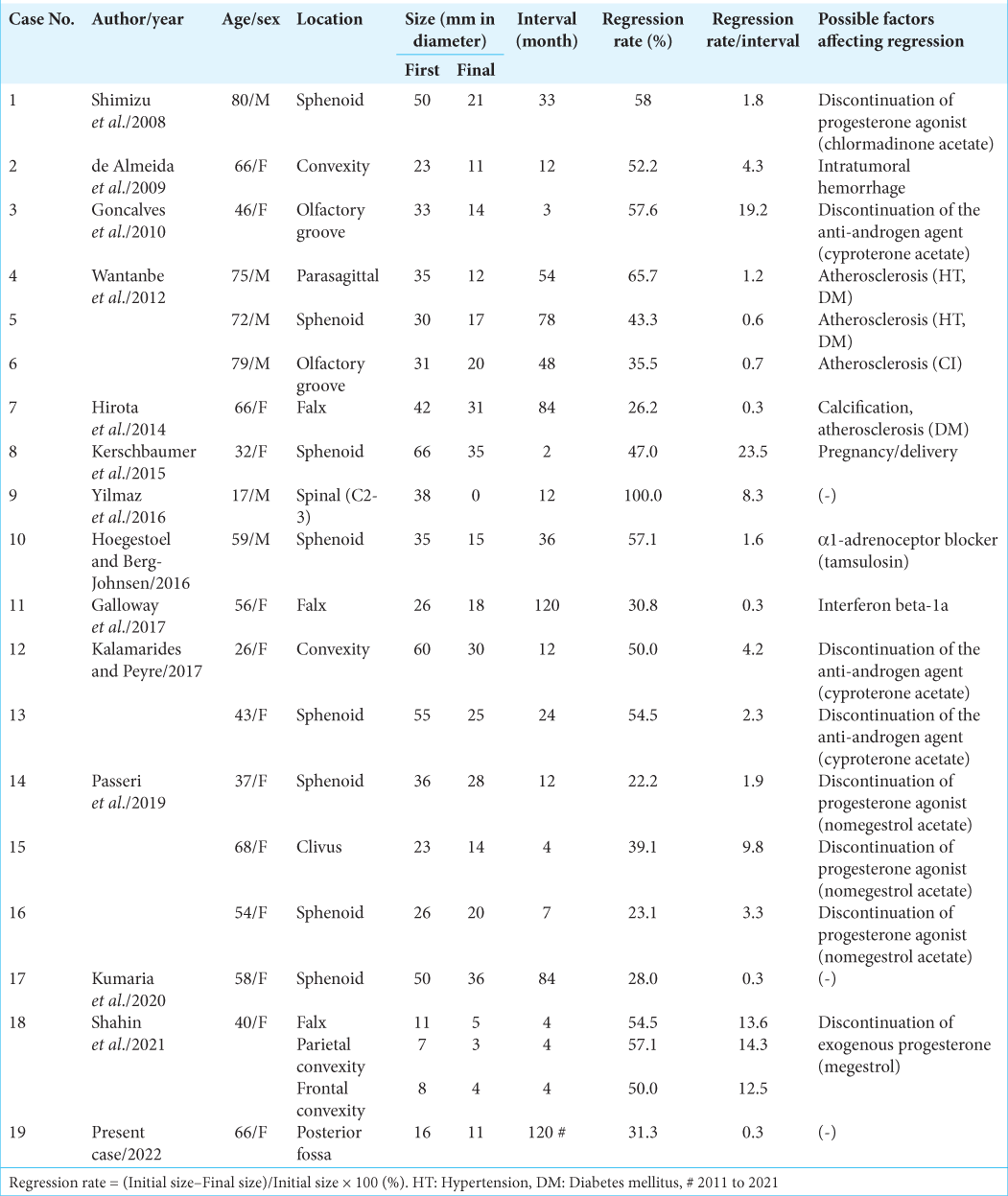
So far, 19 cases (male-female ratio 6:13) of spontaneous meningioma regression, including this case, have been reported [
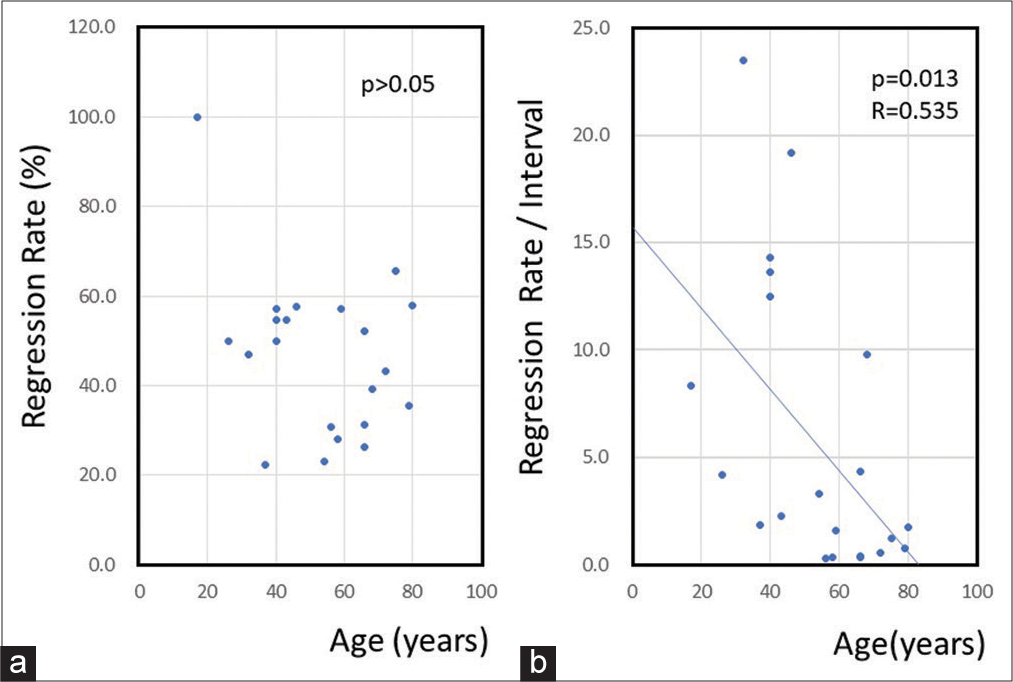
There is little difference between our case and the other reported cases, except for the occurrence site. Our case may be slightly older than other reported cases. When examining patient age and tumor regression, tumor size decreases at all ages, regardless of age [
Figure 4:
(a) A graph showing the relationship between age and tumor regression rate. There is no correlation between age and regression rate. (b) A graph showing the relationship between tumor regression rate and observation interval. The tumor regression rate over a period of time is significantly lower in the elderly (regression analysis, P < 0.05).
Several factors may have caused spontaneous regression, including hormone therapy discontinuation, hormone balance changes associated with pregnancy and delivery, intratumoral hemorrhage, tumor cell calcification promoted by a high glucose level, and atherosclerosis. The involvement of atherosclerosis appears to be conspicuous in the elderly and hormone-related factors seem essential in other age groups. Only three cases, including ours, had little or no known cause.
It has also been reported that the residual tumor disappeared spontaneously after partial resection.[
The mechanism of spontaneous regression in our case is unknown. Depending on the histology of the meningiomas and hormone receptors, there may be types of meningiomas that tend to regress. The cause of regression may be apoptosis, activation of the immune system, or changes in the feeding vessels to the tumor due to aging or atherosclerosis. In this case, however, these are only speculations, as there were no histological diagnoses or changes in the major blood vessels of the brain on MR angiography.
Since meningiomas frequently occur in women, it has long been argued that female hormones are involved in the development and growth of tumors. Meningiomas are 50–80% positive for progesterone receptors and 10–30% positive for estrogen receptors.[
As a limitation of this case report, noncontrast MRI has been used for the follow-up tool. Although noncontrast MRI is reported to be equivalent to postcontrast MRI when following up on changes in meningioma tumor size,[
During tumor follow-up with diagnostic imaging, clinicians should always consider the differential diagnosis. For example, as solitary fibrous tumors resemble meningiomas on imaging but may be more invasive than meningiomas, diffusion-weighted MR imaging with apparent diffusion coefficient mapping has been reported to help differentiate such tumors.[
CONCLUSION
Understanding the natural history of meningiomas is essential for a better selection of treatment approaches or appropriate follow-up. This case may provide new insights into the progression of meningiomas.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Boto J, Guatta R, Fitsiori A, Hofmeister J, Meling TR, Vargas MI. Is contrast medium really needed for follow-up MRI of untreated intracranial meningiomas?. AJNR Am J Neuroradiol. 2021. 42: 1421-8
2. Chikasue T, Uchiyama Y, Tanoue S, Komaki S, Sugita Y, Abe T. Intracranial solitary fibrous tumor/hemangiopericytoma mimicking cystic meningioma: A case report and literature review. Radiol Case Rep. 2021. 16: 1637-42
3. de Almeida JP, Petteys RJ, Sciubba DM, Gallia GL, Brem H. Regression of intracranial meningioma following intratumoral hemorrhage. J Clin Neurosci. 2009. 16: 1246-9
4. Galloway L, Vakili N, Spears J. Spontaneous regression of parafalcine meningioma in a multiple sclerosis patient being treated with interferon beta-1a. Acta Neurochir (Wien). 2017. 159: 469-71
5. Gonçalves AM, Page P, Domigo V, Méder JF, Oppenheim C. Abrupt regression of a meningioma after discontinuation of cyproterone treatment. AJNR Am J Neuroradiol. 2010. 31: 1504-5
6. He JQ, Iv M, Li G, Zhang M, Hayden Gephart M. Noncontrast T2-weighted magnetic resonance imaging sequences for long-term monitoring of asymptomatic convexity meningiomas. World Neurosurg. 2020. 135: e100-5
7. Hirota K, Fujita T, Akagawa H, Onda H, Kasuya H. Spontaneous regression together with increased calcification of incidental meningioma. Surg Neurol Int. 2014. 5: 73
8. Hoegestoel EA, Berg-Johnsen J. Regression of intracranial meningioma during treatment with α1-adrenoceptor blocker. Neurol Surg Rep. 2016. 77: e62-5
9. Hong S, Usami K, Hirokawa D, Ogiwara H. Pediatric meningiomas: A report of 5 cases and review of literature. Childs Nerv Syst. 2019. 35: 2219-25
10. Kalamarides M, Peyre M. Dramatic shrinkage with reduced vascularization of large meningiomas after cessation of progestin treatment. World Neurosurg. 2017. 101: 814.e7-814.e10
11. Kerschbaumer J, Freyschlag CF, Stockhammer G, Taucher S, Maier H, Thomé C. Hormone-dependent shrinkage of a sphenoid wing meningioma after pregnancy: Case report. J Neurosurg. 2016. 124: 137-40
12. Konstantinidou AE, Korkolopoulou P, Mahera H, Kotsiakis X, Hranioti S, Eftychiadis C. Hormone receptors in non-malignant meningiomas correlate with apoptosis, cell proliferation and recurrence-free survival. Histopathology. 2003. 43: 280-90
13. Kumaria A, Ingale HA, Macarthur DC. Spontaneous regression of a large skull base meningioma: Case report. Br J Neurosurg. 2020. 34: 205-6
14. Liu A, Wang JM, Li GL, Sun YL, Sun SB, Luo B. Clinical and pathological analysis of benign brain tumors resected after Gamma Knife surgery. J Neurosurg. 2014. 121: 179-87
15. Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E. Meningioma. Crit Rev Oncol Hematol. 2008. 67: 153-71
16. Matsumoto M, Sanpei K, Ohishi H, Seiki Y, Terao H, Kudo M. Spontaneous regression of a meningioma in Meckel’s cave. Case report. Neurol Med Chir (Tokyo). 1988. 28: 925-9
17. Miyai M, Takenaka K, Hayashi K, Kato M, Uematsu K, Murai H. Effect of an oral anti-estrogen agent (mepitiostane) on the regression of intracranial meningiomas in the elderly. Brain Nerve. 2014. 66: 995-1000
18. Oura S, Sakurai T, Yoshimura G, Tamaki T, Umemura T, Kokawa Y. Regression of a presumed meningioma with the antiestrogen agent mepitiostane. Case report. J Neurosurg. 2000. 93: 132-5
19. Passeri T, Champagne PO, Bernat AL, Hanakita S, Salle H, Mandonnet E. Spontaneous regression of meningiomas after interruption of nomegestrol acetate: A series of three patients. Acta Neurochir (Wien). 2019. 161: 761-5
20. Pravdenkova S, Al-Mefty O, Sawyer J, Husain M. Progesterone and estrogen receptors: Opposing prognostic indicators in meningiomas. J Neurosurg. 2006. 105: 163-73
21. Ricci SB, Cerchiari U. Spontaneous regression of malignant tumors: Importance of the immune system and other factors (Review). Oncol Lett. 2010. 1: 941-5
22. Shahin MN, Bowden SG, Yaghi NK, Bagley JH, Han SJ, Varlamov EV. Regression of multiple meningiomas after discontinuation of chronic hormone therapy: A case report. J Neurol Surg Rep. 2021. 82: e38-42
23. Shimizu J, Matsumoto M, Yamazaki E, Yasue M. Spontaneous regression of an asymptomatic meningioma associated with discontinuation of progesterone agonist administration. Neurol Med Chir (Tokyo). 2008. 48: 227-30
24. Wantanbe S, Tsuchiya D, Kinjo T. Spontaneous regression of meningioma: A report of three cases. Jpn Neurosurg (Tokyo). 2012. 21: 250-4
25. Yilmaz A, Kizilay Z, Sair A, Avcil M, Ozkul A. Spontaneous regression of an incidental spinal meningioma. Open Access Maced J Med Sci. 2016. 4: 128-30
26. Yen YS, Sun LM, Lin CL, Chang SN, Sung FC, Kao CH. Higher risk for meningioma in women with uterine myoma: A nationwide population-based retrospective cohort study. J Neurosurg. 2014. 120: 655-61