- Department of Neurosurgery, Hospital Universitario “Dr. José Eleuterio González” UANL, Monterrey, Nuevo León,
- Department of Neurosurgery, Instituto Nacional de Neurología y Neurocirugía, Ciudad de México, Mexico.
Correspondence Address:
Edgar Nathal, Department of Neurosurgery, Instituto Nacional de Neurología y Neurocirugía, Ciudad de México, Mexico.
DOI:10.25259/SNI_666_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: José A. Arenas-Ruiz1, Nickjail Hernández-Álvarez2, Juan P. Navarro-Garcia de Llano2, Aurelio Ponce-Ayala2, Edgar Nathal2. Spontaneous regression of a thrombosed cerebral arteriovenous malformation in a patient with a prothrombotic state associated with multiple myeloma: A case report and literature review. 19-Oct-2021;12:521
How to cite this URL: José A. Arenas-Ruiz1, Nickjail Hernández-Álvarez2, Juan P. Navarro-Garcia de Llano2, Aurelio Ponce-Ayala2, Edgar Nathal2. Spontaneous regression of a thrombosed cerebral arteriovenous malformation in a patient with a prothrombotic state associated with multiple myeloma: A case report and literature review. 19-Oct-2021;12:521. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11191
Abstract
Background: Cerebral arteriovenous malformations (AVMs) are pathologic communications between veins and arteries of the brain vasculature. Its spontaneous regression is rare, and many factors have been described in the effort to explain this phenomenon, including a hypercoagulable state.
Case Description: We present the case of a spontaneous unruptured AVM regression in a patient where thrombosis of the malformation was found, probably due to a prothrombotic state associated with multiple myeloma (MM).
Conclusion: We aim to contribute to the study of this rare phenomenon, presenting the relationship between a hypercoagulable state caused by MM and the spontaneous AVM regression that has not been previously reported.
Keywords: Cerebral arteriovenous malformation, Multiple myeloma, Prothrombotic state, Spontaneous regression, Vascular neurosurgery
INTRODUCTION
Spontaneous and complete regression of a cerebral arteriovenous malformation (AVM) is an infrequent but well-recognized event, with an estimated prevalence of 0.5–1.3%.[
CASE REPORT
A 49-year-old male was diagnosed in May 2014 with a spontaneous deep venous thrombosis (DVT). Then, treatment with apixaban at a dose of 5 mg twice daily was initiated, suspended 3 months later due to an episode of gastrointestinal bleeding. In September 2014, after a severe headache, an MRI was performed showing a right occipital AVM Spetzler-Martin grade II. Its afferent vessel was the posterior cerebral artery and venous drainage through the right transverse and straight sinus. In November 2014, he was diagnosed with multiple myeloma (MM) IgG kappa, ISS III, and IIIB Durie-Salmon. He received chemotherapy based on 15 cycles of 28 days each with thalidomide 100 mg/day, cyclophosphamide 50 mg/ day, dexamethasone 20 mg weekly (ThaCyDex), and one dose of 4 mg zoledronic acid. Evolution was favorable with complete remission in the following months and protocol for autologous bone marrow transplant was started.
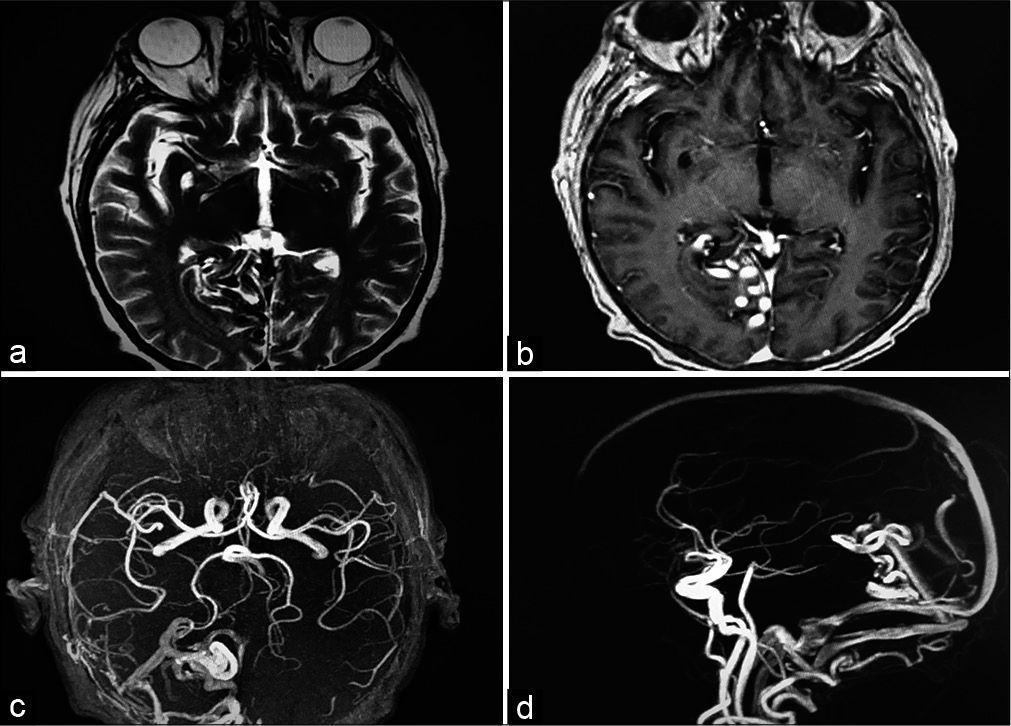
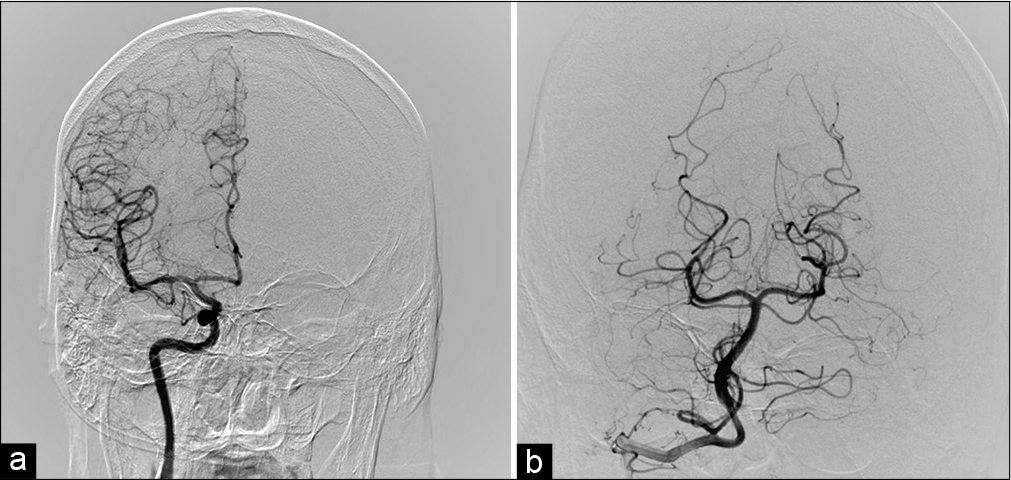
In September 2015, the patient had partial seizures with visual hallucinations, clonic movements of the left limbs, cephalic and eye left version, and secondary generalization. A neurological examination documented left homonymous hemianopia. Nonenhanced head CT scan showed a hyperdense serpentigenous trace in the right parieto-occipital region, with a hypodensity adjacent to this area and without evidence of hemorrhage. An MRI was performed 4 days later and found the same serpentigenous paths shown in the CT scan [
DISCUSSION
The case report illustrates the spontaneous and complete thrombosis of an unruptured cerebral AVM due to a prothrombotic state in a patient with MM. This association is not reported. Multiple theories have been proposed to explain the spontaneous regression of unruptured cerebral AVMs. The main factors postulated include the presence of intravascular turbulence and tortuous venous drainage, single drainage vein and/or single afferent artery, superficial location, small and medium size, and a probable hypercoagulable state.[
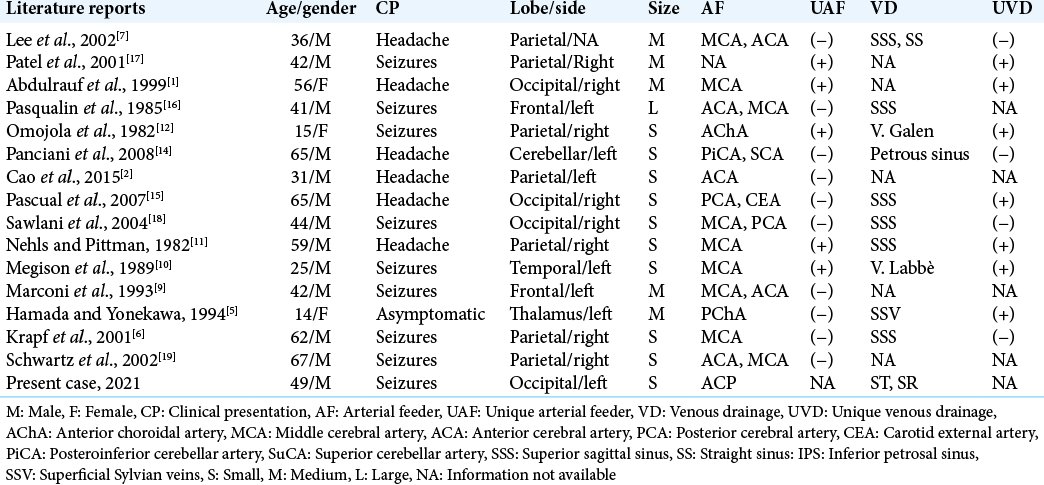
We carried out a literature review of cases of unruptured cerebral AVMs, which showed spontaneous regression without evidence of bleeding or intervention of any kind before obliteration.[
Abdulrauf et al. had a 56-year-old patient with an occipital cerebral AVM that suffered thrombosis and later surgical excised with histological confirmation.[
In the present case, it is evident that the patient carried a prothrombotic state associated with MM; indeed, he had a DVT and was receiving apixaban when he developed the neurological disorder. MM is an oncologic pathology associated with venous and arterial thrombosis, especially in extremities and, in rare cases, pulmonary and cerebral thrombosis.[
CONCLUSION
The relevance of this paper relies on the relationship we describe between the prothrombotic state in a patient with MM and the spontaneous regression of a thrombosed AVM. With this, we hope to contribute to the study of the pathophysiology that can explain this phenomenon in a more precise manner. More studies are needed on the subject, but as far as we are aware, we are the first to expose this relationship between a prothrombotic state, like the one seen in MM, and the spontaneous regression of an AVM, contributing to the understanding of these uncommon but interesting phenomena.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abdulrauf SI, Malik GM, Awad IA. Spontaneous angiographic obliteration of cerebral arteriovenous malformations. Neurosurgery. 1999. 44: 280-7
2. Cao C, Sourour N, Reina V, Nouet A, di Maria F, Chiras J. Spontaneous thrombosis of the main draining vein revealing an unruptured brain arteriovenous malformation. Interv Neuroradiol. 2015. 21: 222-6
3. Cesarman-Maus G, Braggio E, Fonseca R. Thrombosis in multiple myeloma (MM). Hematology. 2012. 17: S177-80
4. Eudo C, Petit A, Mondon K, Rippault H, Dardaine V, Constans T. Cerebral venous thrombosis in an individual with multiple myeloma treated with lenalidomide. J Am Geriatr Soc. 2011. 59: 2371-2
5. Hamada J, Yonekawa Y. Spontaneous disappearance of a cerebral arteriovenous malformation: Case report. Neurosurgery. 1994. 34: 171-3
6. Krapf H, Siekmann R, Freudenstein D, Küker W, Skalej M. Spontaneous occlusion of a cerebral arteriovenous malformation: Angiography and MR imaging follow-up and review of the literature. AJNR Am J Neuroradiol. 2001. 22: 1556-60
7. Lee SK, Vilela P, Willinsky R, TerBrugge KG. Spontaneous regression of cerebral arteriovenous malformations: Clinical and angiographic analysis with review of the literature. Neuroradiology. 2002. 44: 11-6
8. Lenz RA, Saver J. Venous sinus thrombosis in a patient taking thalidomide. Cerebrovasc Dis. 2004. 18: 175-7
9. Marconi F, Parenti G, Puglioli M. Spontaneous regression of intracranial arteriovenous malformation. Surg Neurol. 1993. 39: 385-91
10. Megison P, Batjer HH, Purdy PD, Samson DS. Spontaneous resolution of arteriovenous malformation without hemorrhage. AJNR. Am J Neuroradiol. 1989. 10: 204
11. Nehls DG, Pittman HW. Spontaneous regression of arteriovenous malformations. Neurosurgery. 1982. 11: 776-80
12. Omojola MF, Fox AJ, Viñuela FV, Drake CG. Spontaneous regression of intracranial arteriovenous malformations: Report of three cases. J Neurosurg. 1982. 57: 818-22
13. Ortín X, Rodríguez-Luaces M, Calabuig M, Font L. Stroke in a multiple myeloma patient treated with thalidomide. J Stroke Cerebrovasc Dis. 2006. 15: 283-5
14. Panciani PP, Fontanella M, Carlino C, Bergui M, Ducati A. Progressive spontaneous occlusion of a cerebellar AVM: Pathogenetic hypothesis and review of literature. Clin Neurol Neurosurg. 2008. 110: 502-10
15. Pascual B, Lagares A, Miranda P, Pérez-Núñez A, Arrese I, Lobato RD. Spontaneous regression of cerebral arteriovenous malformations: Case report and review of the literature. Neurocirugia (Astur). 2007. 18: 326-9
16. Pasqualin A, Vivenza C, Rosta L, Scienza R, da Pian R, Colangeli M. Spontaneous disappearance of intracranial arterio-venous malformations. Acta Neurochir (Wien). 1985. 76: 50-7
17. Patel MC, Hodgson TJ, Kemeny AA, Forster DM. Spontaneous obliteration of pial arteriovenous malformations: A review of 27 cases. AJNR Am J Neuroradiol. 2001. 22: 531-6
18. Sawlani V, Handique A, Phadke RV. Spontaneous regression of cerebral AVM due to thrombosis of draining vein-angiographic and MRI demonstration. J Neurol Sci. 2004. 223: 195-8
19. Schwartz ED, Hurst RW, Sinson G, Bagley LJ. Complete regression of intracranial arteriovenous malformations. Surg Neurol. 2002. 58: 139-47