- Department of Neurosurgery, Cannizzaro Hospital, Sicily, Italy.
- Department of Neurosurgery, Highly Specialized Hospital and of National Importance “Garibaldi,” Sicily, Italy.
- Plastic Surgery Unit, Cannizzaro Hospital, Sicily, Italy.
- Department of Pathological Anatomy, Cannizzaro Hospital, Sicily, Italy.
- Department of Advanced Technologies, Nuclear Medicine and PET, Cannizzaro Hospital, Sicily, Italy.
- Medical Physics Unit, Cannizzaro Hospital, Catania, Sicily, Italy.
Correspondence Address:
Giuseppe Emmanuele Umana, Department of Neurosurgery, Cannizzaro Hospital, Catania, Sicily, Italy.
DOI:10.25259/SNI_917_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Giuseppe Emmanuele Umana1, Gianluca Scalia2, Paolo Palmisciano1, Maurizio Passanisi2, Gianluca Pompili3, Paolo Amico4, Massimo Ippolito5, Maria Gabriella Sabini6, Salvatore Cicero1, Rosario Perrotta3. Spontaneous sacral fracture with associated acrometastasis of the hand. 25-Oct-2021;12:534
How to cite this URL: Giuseppe Emmanuele Umana1, Gianluca Scalia2, Paolo Palmisciano1, Maurizio Passanisi2, Gianluca Pompili3, Paolo Amico4, Massimo Ippolito5, Maria Gabriella Sabini6, Salvatore Cicero1, Rosario Perrotta3. Spontaneous sacral fracture with associated acrometastasis of the hand. 25-Oct-2021;12:534. Available from: https://surgicalneurologyint.com/surgicalint-articles/11202/
Abstract
Background: Acrometastases, secondary tumors affecting oncological patients with systemic metastases, are associated with a poor prognosis. In rare cases, acrometastases may precede establishing the primary tumor diagnosis.
Case Description: A 72-year-old female heavy smoker presented with low back pain, and right lower extremity sciatica/radiculopathy. X-rays, CT, MR, and PET-CT scans documented primary lung cancer with multi-organ metastases and accompanying pathological fractures involving the sacrum (S1) and right 4th digit. She underwent a S1 laminectomy and amputation of the distal phalanx of the right fourth finger. The histological examination documented a poorly differentiated pulmonary adenocarcinoma infiltrating bone and soft tissues in the respective locations. The patient was treated with a course of systemic immunotherapy (i.e. pembrolizumab). At 6-month follow-up, the patient is doing well and can stand and walk without pain.
Conclusion: Spontaneous sacral fractures may be readily misdiagnosed as osteoporotic and/or traumatic lesions. However, in this case, the additional simultaneous presence of a lytic finger lesion raised the suspicion that these were both metastatic tumors. Such acrometastases, as in this case attributed to a lung primary, may indeed involve the spine.
Keywords: Acrometastases, Elderly, Hand metastases, Immunotherapy, Sacral fracture, Spine surgery
INTRODUCTION
Acrometastases comprise the 0.1% of all bone metastases, including those found in the spine.[
CASE ILLUSTRATION
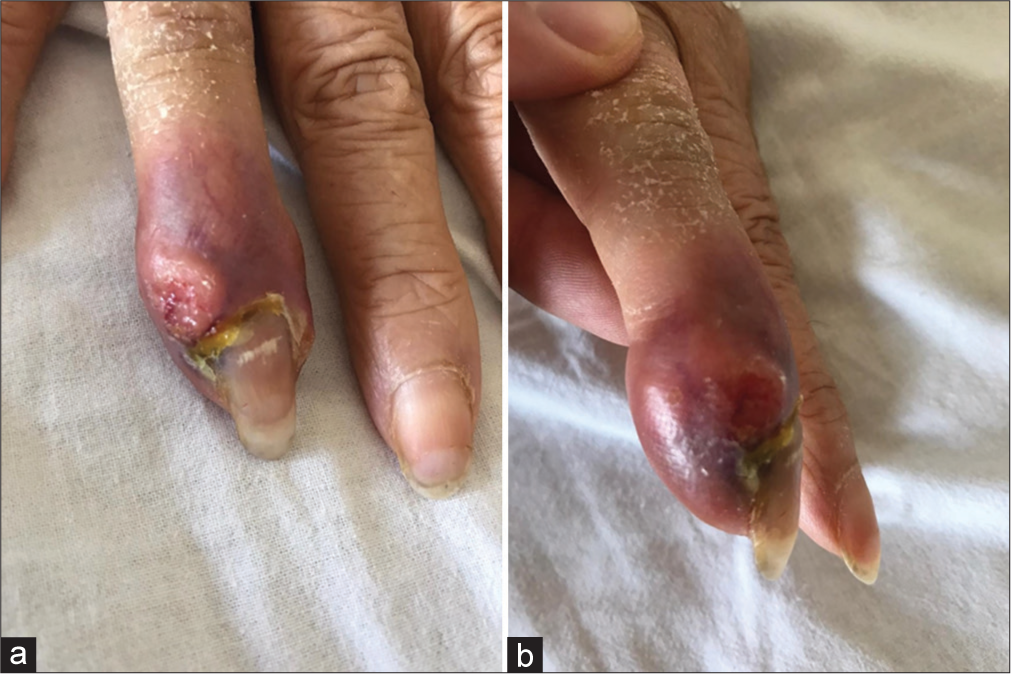
A 72-year-old heavy female smoker, with no history of trauma, presented with 20 days of low back pain, right lower extremity sciatica, and a lesion of the right 4th digit. On examination, the patient had right S1 motor/sensory deficits, and ecchymosis/ swelling of the distal phalanx of the right fourth digit [
Diagnostic evaluations
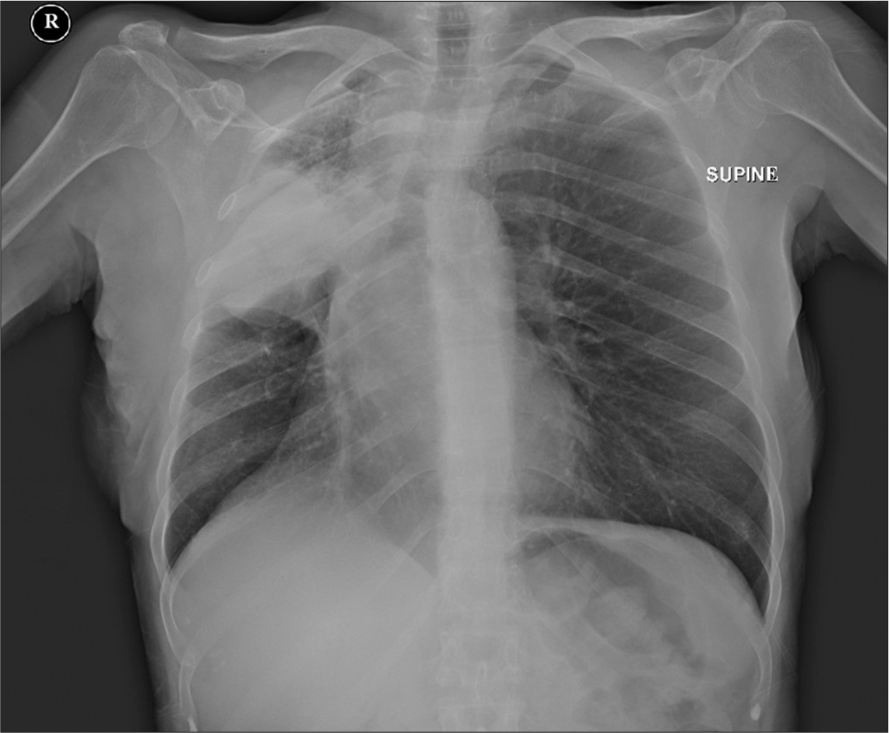
Chest X-ray
The chest X-ray revealed a radiopaque lesion in the right mid-upper lung, with atelectasis and slight retraction of the superior mediastinum [
Hand X-ray
The hand X-ray showed soft tissue thickening of the right ring finger’s distal phalanx, with a distal phalanx fracture and bone loss/lytic process.
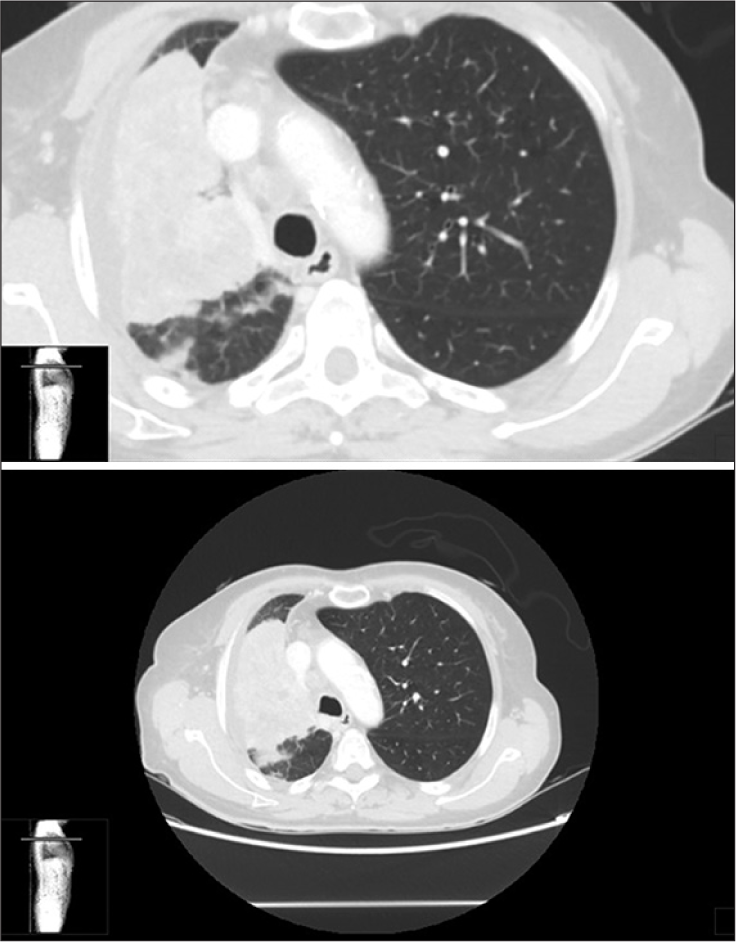
Full body CT
The full-body CT scan documented a large thoracic mass with inhomogeneous contrast enhancement. There were also hypodense areas in the anterior segment of the right upper lobe (90 × 84 mm), with infiltration of the bronchial and vascular branches. An adjacent nodular lesion (11 × 11 mm) and an additional opacity were distinguished in the lateral basal segment of the left lower lobe (5 × 5 mm; considered satellite metastases). Several lymphadenopathies were appreciable in multiple thoracic locations. Osteolytic areas with fractures were detected in the right pubic ramus and at S1 [
PET-CT
The 18F-FDG PET-CT scan showed accumulation of tracer at multiple sites: in the right adrenal gland (SUV max 11.2), in the middle and upper lung lobes (SUV max 15.8), and in several thoracic lymph nodes (SUV max 16.6). Tracer uptake was also found at T7, T8, T10, L3, the sacrum, the right acetabulum and ischiopubic branch, and the fourth finger of the right hand (SUV max 18.3).
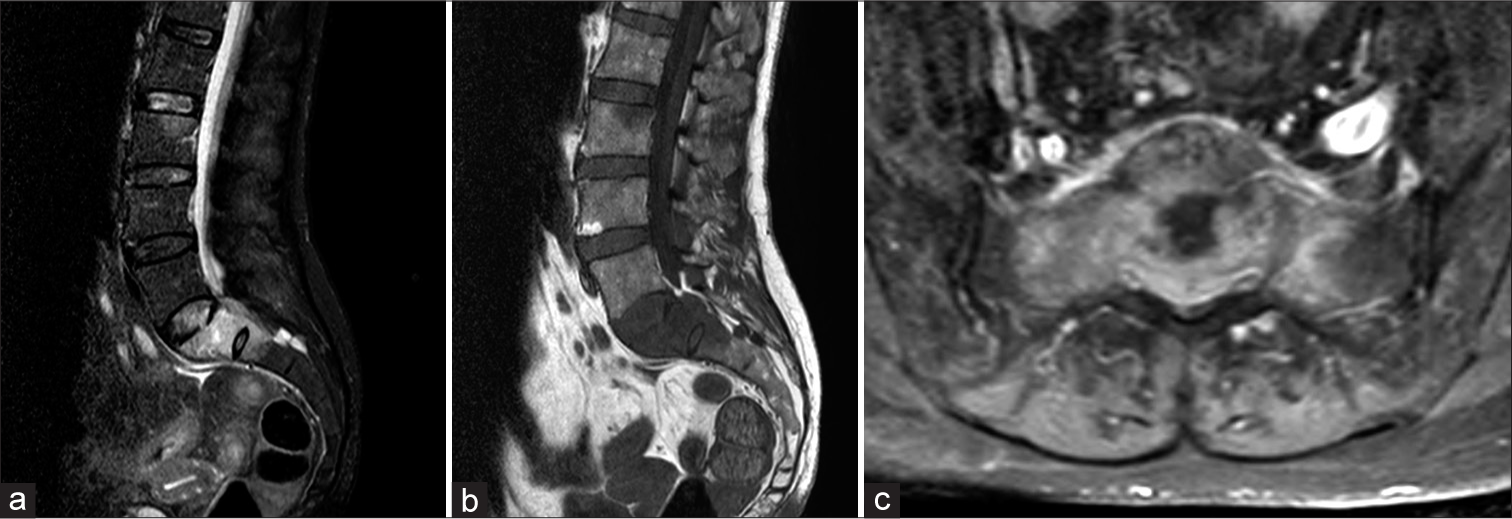
Lumbar MR
The lumbar MRI study revealed morphological alterations involving the L3, S1, and S2 levels that markedly homogeneously enhanced with contrast [
Figure 4:
(a-c) Lumbosacral MR scan showing S1 fracture with hyperintense S1 and S2 vertebral bodies on sagittal STIR images. Lumbosacral MRI study with gadolinium enhancement showing morpho-structural alteration of the bodies of S1 (also affecting the wings) and S2, with conspicuous contrast-enhancement.
Surgery at S1 and removal of distal phalanx right fourth digit
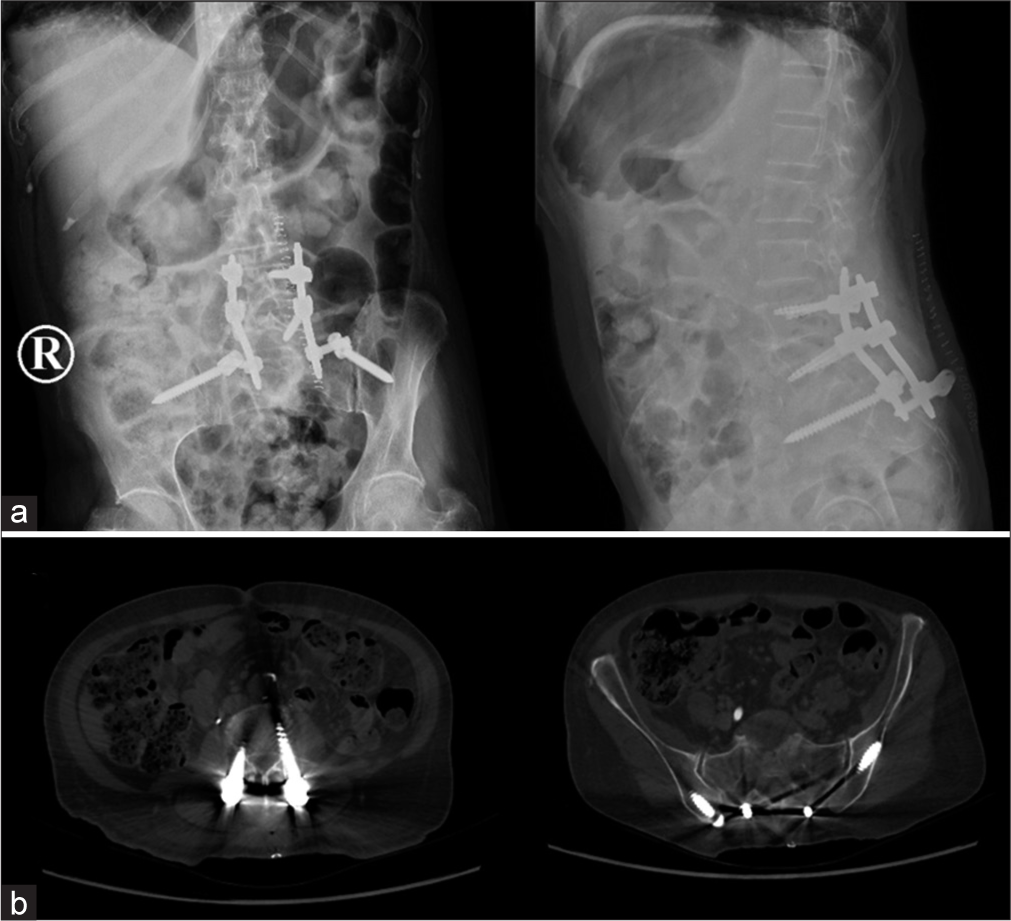
The patient underwent a S1 decompressive laminectomy with concurrent biopsy of the S1 vertebral body, lumbopelvic fixation (L4-L5-ileum), and thermal ablation of the L3 lesion. The distal phalanx of the right fourth finger was also amputated.
Postoperative course
The patient recovered postoperatively, without any complications, and was able to stand/walk after 2 weeks of rehabilitation. The postoperative CT scan documented adequate neural decompression and screw placement [
Histology
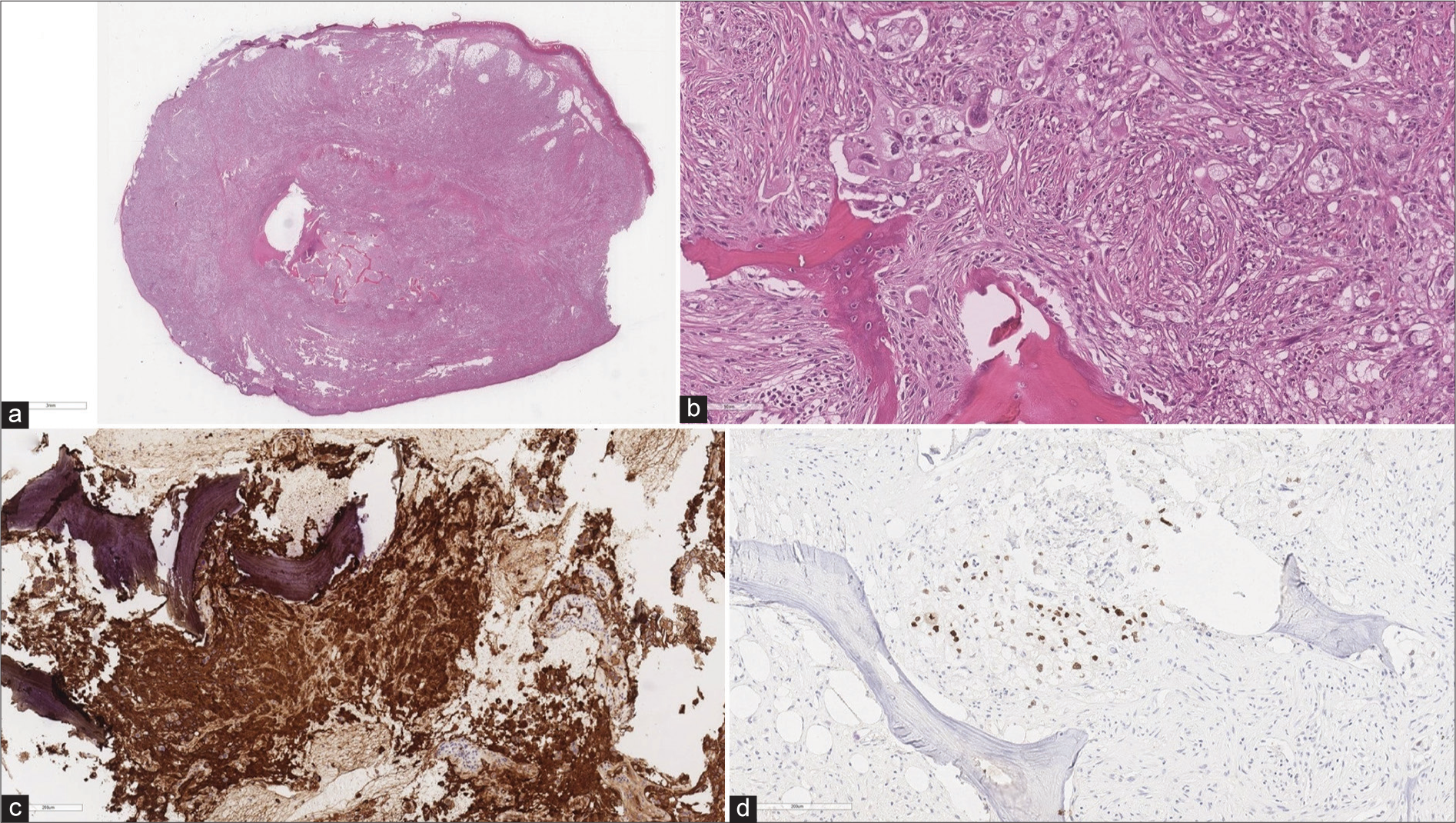
The histological examination revealed a poorly differentiated pulmonary adenocarcinoma infiltrating the bone and soft tissues at S1 and the 4th digit. Immunohistochemical stains were positive for: CKAE1/AE3, CK7, EMA, vimentin, and TTF1 [
Figure 6:
Histologic examination showing (a) widespread bone and soft tissue infiltration by poorly differentiated neoplasm (E-E) (a), (b) bone infiltration by neoplasm consisting of cells with large eo-sinophilic and clear cytoplasm, vesicular nucleus, and small nucleolus (E and E), (c) diffuse and strong immunostaining for CK7, (d) neoplastic cells immunopositive for TTF-1.
Immunotherapy and radiotherapy
The patient received a course of immunotherapy (pembrolizumab 400 mg IV/42 days) and targeted radiotherapy (20 Gy/4 Gy/fraction) to treat the osteolytic lesions in the right pubic ramus.
Clinical outcome at 6 postoperative months
At 6-month follow-up, the patient can stand and walk without any pain. However, she reports the new onset of transient diplopia, related to the lesion in the left orbit, which regresses with corticosteroids. The last full-body PET scan showed reduced tracer uptake in previous lesions, but also new secondary lesions in the left lung, frontal lobe, parietal lobe, and C6 vertebral body.
DISCUSSION
Acrometastases characterize a heterogeneous group of rare secondary tumors.[
While X-ray often shows non-specific osteolytic lesions of the affected finger, PET scans have a 90% sensitivity and 78% specificity in detecting benign and malignant tumor.[
CONCLUSION
The present case suggests that, despite the poor prognosis of patients with acrometastases, systemic immunotherapy may prolong survival, thus enabling surgical spine decompression and fixation with the goal of improving the patient’s quality of life.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Afshar A, Farhadnia P, Khalkhali H. Metastases to the hand and wrist: An analysis of 221 cases. J Hand Surg Am. 2014. 39: 923-32.e17
2. Hayden RJ, Sullivan LG, Jebson PJ. The hand in metastatic disease and acral manifestations of paraneoplastic syndromes. Hand Clin. 2004. 20: 335-43
3. Umana GE, Scalia G, Palmisciano P, Passanisi M, da Ros V, Pompili G. Acrometastases to the hand: A systematic review. Medicina (Kaunas). 2021. 57: 950