- Department of Neurosurgery, Hannover Medical School, Hannover, Germany.
- Department of Haematology, Haemostaseology, Oncology and Stem Cell Transplantation, Hannover Medical School, Hannover, Germany.
- Department of Neuropathology, Hannover Medical School, Hannover, Germany.
- Department of Institute for Diagnostic and Interventional Neuroradiology, Hannover Medical School, Hannover, Germany.
Correspondence Address:
F. H. Hounchonou, Department of Neurosurgery, Hannover Medical School, Hannover, Germany.
DOI:10.25259/SNI_576_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: F. H. Hounchonou1, Joachim Runge1, Arnold Ganser2, Christian Hartmann3, Peter Raab4, Joachim K. Krauss1. Stereotactic biopsy of a brain lesion caused by hormographiella aspergillata. 30-Dec-2022;13:596
How to cite this URL: F. H. Hounchonou1, Joachim Runge1, Arnold Ganser2, Christian Hartmann3, Peter Raab4, Joachim K. Krauss1. Stereotactic biopsy of a brain lesion caused by hormographiella aspergillata. 30-Dec-2022;13:596. Available from: https://surgicalneurologyint.com/surgicalint-articles/12087/
Abstract
Background: Invasive fungal infections are an increasing problem in immunosuppressed patients. In patients with the central nervous system involvement, there is a high case fatality rate. There is a very limited experience with infections caused by Hormographiella aspergillata (HA) in such cases and most often diagnosis is only confirmed postmortem.
Case Description: We report the case of a 53-year-old woman with acute myeloid leukemia. After primary therapy with daunorubicin, cytarabine, and gemtuzumab ozogamicin, the patient developed pneumonia and later neurological symptoms caused by multiple gadolinium-enhancing brain lesions in magnetic resonance imaging (MRI). Stereotactic biopsy of a frontal precentral lesion was performed and revealed HA infection. The patient died in the further course secondary to cardiopulmonary problems.
Conclusion: Stereotactic biopsy is a safe way to establish the diagnosis of unclear lesions such as HA infection. We recommend to perform stereotactic biopsy early in immunocompromised patients with brain lesions to guide further treatment.
Keywords: Cerebral fungal infection, Coprinopsis cinerea, Hematopoietic stem cell transplantation, Hormographiella aspergillata, Invasive fungal infection, Stereotactic biopsy
INTRODUCTION
Invasive fungal infections (IFI) are an increasing problem regarding the growing number of people at risk. They cause more than 1.5 million deaths globally every year.[
Hormographiella aspergillata (HA) is known as the asexual form of Coprinopsis cinerea which is a species of the basidiomycete mushroom genus.[
CLINICAL PRESENTATION
A 53-year-old woman was admitted to Hannover Medical School after developing left-sided paresthesias and hemiparesis. Her medical history was remarkable for high-risk AML, which had been diagnosed 2 months earlier, with initially over 80% of blasts in bone marrow. In addition, she suffered from a chronic anxiety disorder and depression with a history of tranquilizer and antidepressant drugs intake.
The primary therapy had consisted in medication with daunorubicin, cytarabine, and gemtuzumab ozogamicin (DA + GO). On day 15, a good response to therapy was registered (<5% blasts in bone marrow). Nevertheless, the patient had a persistent cytopenia with prominent leukopenia (up to 0.1 103/µL). Thereafter, she developed pneumonia with recurring fever and an increasing CRP (up to 430 mg/L). A computed tomography (CT) scan of the chest performed on day 35 showed multiple nodules in both lungs, matching with a fungal pneumonia. Bronchoalveolar lavage (BAL) cultures however tested negative for fungi and tuberculosis. In particular, no Aspergillus antigen could be detected. Initial antifungal prophylaxis with Voriconazole was switched to Caspofungin. Subsequent bone marrow examination revealed no relapse of AML.
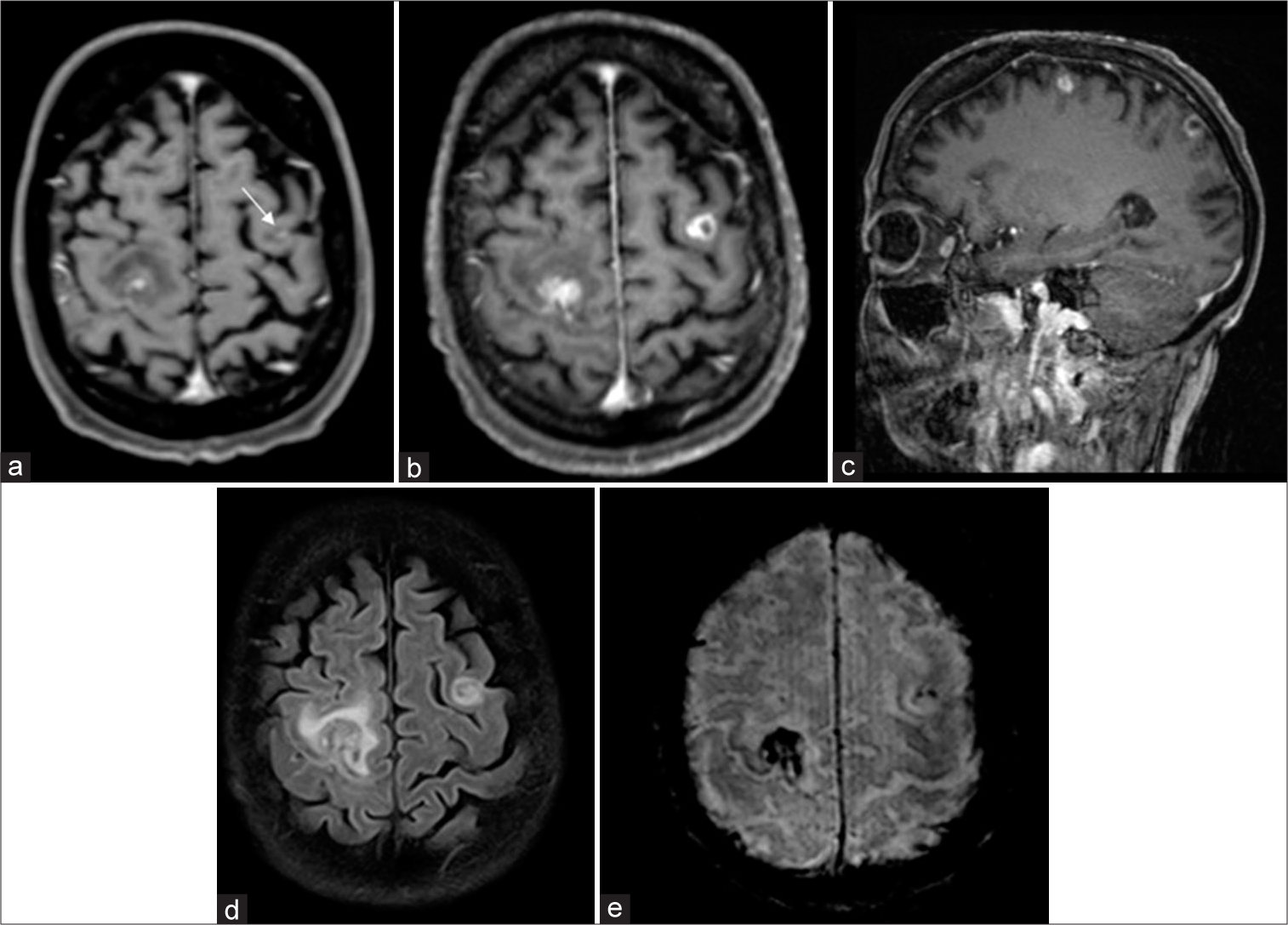
After the occurrence of paresthesias and hemiparesis, brain magnetic resonance imaging (MRI) showed multiple gadolinium-enhancing cerebral lesions [
Figure 1:
Brain magnetic resonance imaging findings of a 53-year-old woman with acute myeloid leukemia and multifocal cerebral lesions caused by Hormographiella aspergillata: (a) initial axial post contrast T1-weighted scan shows two gadolinium enhancing lesions in both frontal hemispheres with minor mass effect on the right side with a leptomeningeal enhancement on the left (arrow). (b-e) Follow-up imaging: (b) axial post contrast T1-weighted scan displays the progression of the gadolinium enhancing lesions compared to (a and c) sagittal post contrast T1-weighted scan showing a frontal as well as parietal lesion with the same lesion characteristic as in (a-d) The FLAIR image shows the amount of surrounding edema with different extent between the two lesions. (e) Susceptibility weighted imaging demonstrates signal loss due to paramagnetic effects caused by iron deposition/microhemorrhages.
On admission, the patient was in a poor general condition. She was obtunded and had a marked left-sided hemiparesis. Stereotactic serial biopsies were obtained (Riechert Mundinger frame with Zamorano-Dujovny application) targeting the right precentral lesion under general anesthesia. The collected samples were sent for microbiological and neuropathologic analyses. After surgery, the patient was transferred to the intensive care unit. Postoperative CT scan was unremarkable. Nevertheless, the patient had increasing cardiorespiratory problems, which led to her death on day 70. Autopsy was declined by her family.
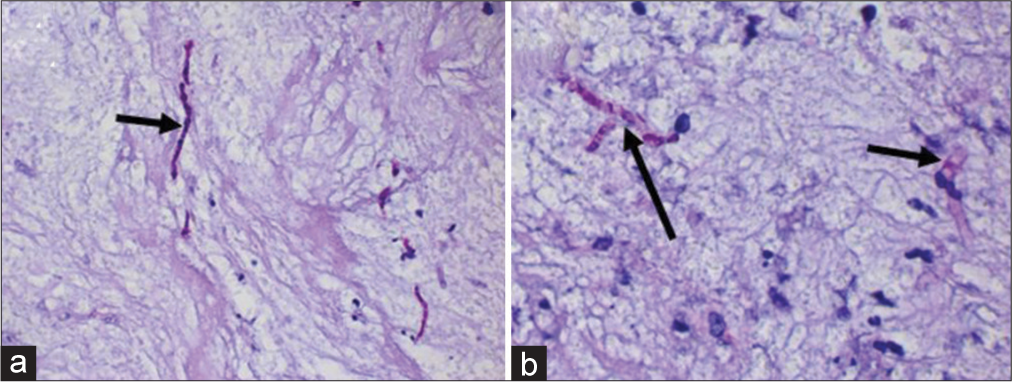
Microscopic examination of the stereotactic biopsy specimen revealed gliotic central nervous system (CNS) parenchyma with sparse lymphomonocytic infiltrates. In addition, there were necrotic areas. Within the necrotic zones, fungal hyphae were detected by periodic acid Schiff (PAS) staining [
Figure 2:
Microscopic examination of the stereotactic biopsy of the right-sided frontal lesion. Periodic Acid Schiff staining. (a) Hyphae with a string-like appearance and bulbous beads (arrow) on the background of necrotic CNS parenchyma; (b) focally branching hyphae (long arrow) and partly septate hyphae (short arrow) can be identified. Only single lymphocytes and macrophages are found within the necrosis.
DISCUSSION
Stereotactic biopsy in fungal brain infections
Stereotactic cerebral biopsy is a routine neurosurgical procedure with a diagnostic yield of more than 90% at a relatively low risk, which allows to obtain specimen of lesions before tailored therapy. Alternative options are frameless or neuronavigated techniques. Stereotactic biopsy is mostly performed in patients with deep-seated lesions, unresectable lesions, or in tumors suspected to be radio- or chemosensitive such as cerebral lymphoma or germinoma.[
Fungal cerebral infections
Fungal infections of the central nervous system are rare conditions but associated with high lethality, especially in immunocompromised patients. Common responsible fungi in CNS infections are Candida spp., Cryptococcus neoformans, Aspergillus spp., or Mucoromycetes spp.[
The clinical presentation of fungal infections of the CNS is variable and may include meningitis, meningoencephalitis, stroke, cerebritis, vasculitis, and venous sinus thrombosis. Frequent symptoms are headache, fever, seizures, motor symptoms, progressive confusion, altered mental status, nausea, vomiting, visual impairment, papilledema, and focal neurological deficits.[
Radiological findings in fungal CNS infections are non-specific and vary from fungal meningitis, fungal cerebritis, brain abscess, and cryptococcoma to meningeal vasculitis.[
Cerebral infections caused by HA
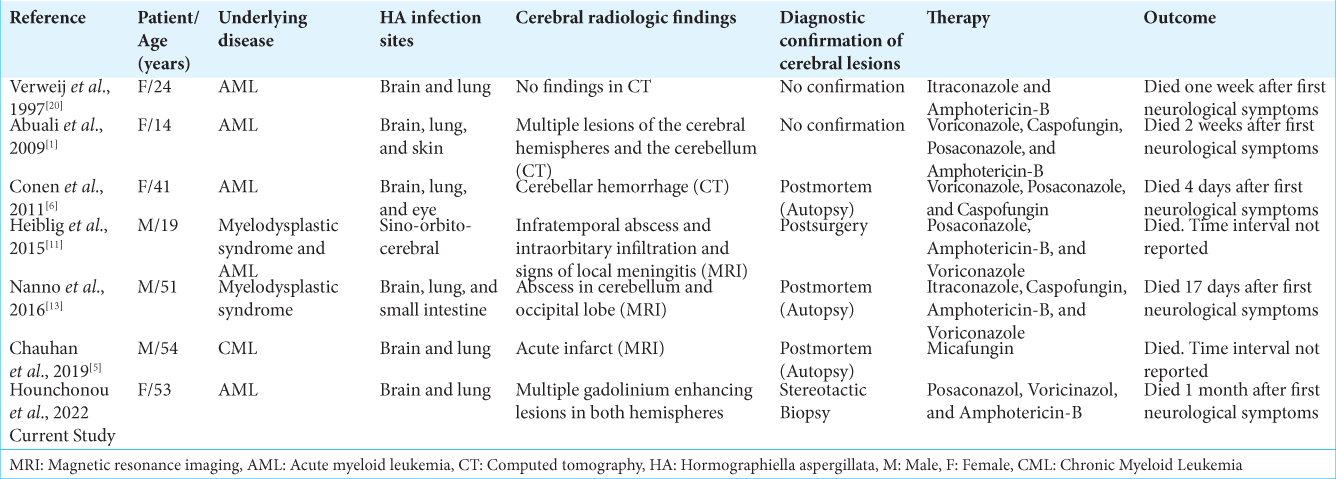
HA is a largely unknown pathogen and it is rarely identified. The number of documented cases is low and thus, no definitive treatment has been specified. Only six cases of cerebral infection related to HA have been reported until now [
All these patients were immunosuppressed, aged between 14 and 54 years, and had underlying hematological malignancies (acute myeloid leukemia, chronic myeloid leukemia, or myelodysplastic syndrome). Most patients underwent hematopoietic stem cell transplantation. Neurological manifestations included epileptic seizures, anosmia, amaurosis, signs of intracranial hypertension, altered mental status, tingling, and hemiparesis. Radiologic examinations showed unspecific findings. In two cases, MRI findings corresponded to brain abscesses.[
Since clinical and radiological findings were unspecific and histological examinations were not available, a definitive confirmation of cerebral HA infection could be confirmed only postmortem in the majority of the published patients.
Therapeutic interventions were based on administration of common antifungal drugs, namely, Posaconazole, Voriconazole, Caspofungin, Micafungin, and Amphotericin-B in various constellations. In all reported cases and in the patient reported here, the infection was fatal. Patients died within a period of 4 weeks after onset of neurological symptoms.[
CONCLUSION
HA brain infection is a rare condition with unspecific clinical und radiological findings, which makes definitive diagnosis challenging. We here show that stereotactic biopsy and neuropathologic examination of the cerebral lesions is a valid option to establish the diagnosis of HA brain infection in the living patient. We strongly recommend to perform stereotactic biopsy early and more frequently in immunocompromised patients with unclear brain lesions. Such a regime allows to tailor treatment more specifically and might result ultimately in a better survival of these severely ill patients.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abuali MM, Posada R, Del Toro G, Roman E, Ramani R, Chaturvedi S. Rhizomucor variabilis var. regularior and Hormographiella aspergillata infections in a Leukemic Bone marrow transplant recipient with refractory neutropenia. J Clin Microbiol. 2009. 47: 4176-9
2. Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev. 2014. 27: 490-526
3. Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel). 2017. 3: 57
4. Brumble LM, Reza MB, Dhakal LP, Cruz G, Saleh OM, Heckman . Fungal infections of the central nervous system: Clinical, radiographic and laboratory manifestations. J Microbial Exp. 2017. 5: 00167
5. Chauhan A, Gruenberg J, Arbefeville S, Mettler T, Brent CH, Ferrieri P. Disseminated Hormographiella aspergillata infection with lung and brain involvement after allogenic hematopoietic stem-cell transplantation in a 54-Year-old man. Lab Med. 2019. 50: 426-31
6. Conen A, Weisser M, Hohler D, Frei R, Stern M. Hormographiella aspergillata An emerging mould in acute leukaemia patients?. Clin Microbiol Infect. 2011. 17: 273-7
7. Gavito-Higuera J, Mullins CB, Ramos-Duran L, Chacon CI, Hakim N, Palacios E. Fungal infections of the central nervous system: A pictorial review. J Clin Imaging Sci. 2016. 6: 24
8. Geno J, Guillamon JM, Guarro J, Pujol I, Ulfig K. Molecular characterization, relatedness and antifungal susceptibility of the basidiomycetous hormographiella species and Coprinus cinereus from clinical and environmental sources. Antonie Van Leeuwenhoek. 1996. 70: 49-57
9. Góralska K, Blaszkowska J, Dzikowiec M. Neuroinfections caused by fungi. Infection. 2018. 46: 443-59
10. Guarro J, Gene J, De Vroey C, Gueho E. Hormographiella; a new genus of hyphomycetes from clinical sources. Mycotaxon. 1992. 45: 179-90
11. Heiblig M, Bozzoli V, Saison J, Thomas X, De Croze D, Traverse-Glehen A. Combined medico-surgical strategy for invasive sino-orbito-cerebral breakthrough fungal infection with Hormographiella aspergillata in an acute leukaemia patient. Mycoses. 2015. 58: 308-12
12. Luzzati R, Ferrari S, Nicolato A, Piovan E, Malena M, Merighi M. Stereotactic brain biopsy in human immunodeficiency virus-infected patients. Arch Intern Med. 1996. 56: 565-8
13. Nanno S, Nakane T, Okamura H, Nishimoto M, Koh H, Nakamae H. Disseminated Hormographiella aspergillata infection with involvement of the lung, brain, and small intestine following allogeneic hematopoietic stem cell transplantation: case report and literature review. Transplant Infect Dis. 2016. 18: 611-6
14. Neves N, Santos L, Reis C, Sarmento A. Candida albicans brain abscesses in an injection drug user patient: A case report. BMC Res Notes. 2014. 7: 837
15. Pathakumari B, Liang G, Liu W. Immune defence to invasive fungal infections: A comprehensive review. Biomed Pharmacother. 2020. 130: 110550
16. Pfaller MA, Pappas PG, Wingard JR. Invasive fungal pathogens: Current epidemiological trends. Clin Infect Dis. 2006. 43: 3-14
17. Reithmeier T, Lopez WO, Doostkam S, Machein MR, Pinsker MO, Trippel M. Intraindividual comparison of histopathological diagnosis obtained by stereotactic serial biopsy to open surgical resection specimen in patients with intracranial tumours. Clin Neurol Neurosurg. 2013. 115: 1955-60
18. Sharma RR. Fungal infections of the nervous system: Current perspective and controversies in management. Int J Surg. 2010. 8: 591-601
19. Slomka M, Doub J. A rare case of Blastomyces dermatitidis brain abscess in an immunocompetent host. Med Mycol Case Rep. 2020. 18: 8-11
20. Verweij PE, van Kasteren M, van de Nes J, de Hoog GS, de Pauw BE, Meis JF. Fatal pulmonary infection caused by the basidiomycete Hormographiella aspergillata. J Clin Microbiol. 1997. 35: 2675-8
21. Zhu Z, Huang Z, Li Z, Li X, Du C, Tian Y. Multiple brain abscesses caused by infection with Candida glabrata A case report. Exp Ther Med. 2018. 15: 2374-80