- Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama, United States,
- Department of School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, United States,
- Department of Radiation Oncology, University of Alabama at Birmingham, Birmingham, Alabama, United States.
Correspondence Address:
James Mooney, Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama, United States.
DOI:10.25259/SNI_86_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: James Mooney1, Arsalaan Salehani1, Nicholas Erickson1, Evan Thomas3, Adeel Ilyas1, Sage Rahm1, Nicholas Eustace2, Pedram Maleknia2, Omer Yousuf2, Markus Bredel3, John Fiveash3, Chris Dobelbower3, Winfield Fisher1. Stereotactic radiosurgery for ruptured versus unruptured intracranial arteriovenous malformations. 06-May-2022;13:194
How to cite this URL: James Mooney1, Arsalaan Salehani1, Nicholas Erickson1, Evan Thomas3, Adeel Ilyas1, Sage Rahm1, Nicholas Eustace2, Pedram Maleknia2, Omer Yousuf2, Markus Bredel3, John Fiveash3, Chris Dobelbower3, Winfield Fisher1. Stereotactic radiosurgery for ruptured versus unruptured intracranial arteriovenous malformations. 06-May-2022;13:194. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11578
Abstract
Background: There are a limited data examining the effects of prior hemorrhage on outcomes after stereotactic radiosurgery (SRS). The goal of this study was to identify risk factors for arteriovenous malformation (AVM) rupture and compare outcomes, including post-SRS hemorrhage, between patients presenting with ruptured and unruptured AVMs.
Methods: A retrospective review of consecutive patients undergoing SRS for intracranial AVMs between 2009 and 2019 at our institution was conducted. Chi-square and multivariable logistic regression analyses were utilized to identify patient and AVM factors associated with AVM rupture at presentation and outcomes after SRS including the development of recurrent hemorrhage in both ruptured and unruptured groups.
Results: Of 210 consecutive patients with intracranial AVMs treated with SRS, 73 patients (34.8%) presented with AVM rupture. Factors associated with AVM rupture included smaller AVM diameter, deep venous drainage, cerebellar location, and the presence of intranidal aneurysms (P P = 0.51). There were no significant differences in obliteration rate, time to obliteration, or adverse effects requiring surgery or steroids between unruptured and ruptured groups.
Conclusion: Smaller AVM size, deep venous drainage, and associated intranidal aneurysms were associated with rupture at presentation. AVM rupture at presentation was not associated with an increased risk of recurrent hemorrhage or other complication after SRS when compared to unruptured AVM presentation. Obliteration rates were similar between ruptured and unruptured groups.
Keywords: Arteriovenous malformation, Post-radiosurgery hemorrhage, Rupture, Stereotactic radiosurgery
INTRODUCTION
Cerebral arteriovenous malformations (AVMs) are comprised shunting between abnormal arteries and veins with an intervening nidus.[
Given the increased risk of ICH after initial AVM rupture, SRS has not been traditionally considered a preferred treatment modality in ruptured AVM patients. Although AVM obliteration rates were largely similar between the ruptured and unruptured groups, Ding et al. found that prior rupture significantly increased the annual risk of post-SRS hemorrhage in the latency period before nidus obliteration.[
We contribute a single-center and retrospective study to this growing body of the literature, specifically investigating factors associated with AVM rupture and whether rupture is associated with an increased risk of complications including recurrent hemorrhage after SRS.
MATERIALS AND METHODS
The medical records of 210 consecutive patients undergoing SRS for intracranial AVMs between December 2009 and December 2019 were reviewed retrospectively. Institutional review board approval was obtained before review. Patient characteristics including demographics, comorbidities, and presenting symptoms including AVM ruptured versus unruptured presentation were recorded in addition to AVM characteristics including Spetzler-Martin (SM) grade, size, eloquence, deep venous drainage, location, and the presence of intranidal aneurysms. Post-SRS data including AVM obliteration, posttreatment hemorrhage, adverse effect requiring steroids, and adverse effect requiring surgery were also recorded when available. Diagnosis of AVM was made in all cases using computed tomography angiography (CTA), magnetic resonance angiography, or digital subtraction angiography (DSA). The post-SRS scan type demonstrating obliteration, the time to obliteration, and the presence of peri-nidal T2 signal increase/ edema on post-SRS MRI were also recorded. The decision to pursue SRS was made on a case basis. SRS is preferentially used for AVMs in deep or critical brain regions, in patients with an unacceptable risk of perioperative complications as well as for those refusing to undergo craniotomy. Open surgeries are more often performed in patients with significant life-threatening hemorrhage resulting in mass effect or in cases where AVMs presented to the surface in noneloquent locations.
Radiosurgery
All radiosurgical cases were performed using the Leksell Gamma Knife with Gamma Plan software (Elekta AB) or with the linear accelerator (LINAC). The AVM nidus was defined using magnetic resonance imaging (MRI) and CTA and supplemented with DSA in select cases. The decision to perform SRS was determined based on a combination of patient factors including age and medical comorbidities as well as AVM factors such as larger size and location that may have predisposed a patient to higher risk with open surgery. The margin SRS dose was constructed to include the entire volume of the AVM. A dose of 17.5 Gy (range 17.5–18.5) to the 50% (range 40–70%) isodose line was the most commonly used treatment plan.
Statistical analysis
All statistical analysis was carried out in SPSS 27 (IBM Corp. Armonk, NY). A t-test was used to compare continuous variables across groups. Pearson’s Chi-square or Fisher’s exact tests were used for comparisons of categorical variables, depending on expected cell count. Binary logistic regression analysis was utilized when examining interval independent variables with associated binary categorical dependent variables. The significance level was set at ≤0.05 and a two-sided probability testing was used.
RESULTS
Study cohort: Patient and AVM factors associated with AVM Rupture
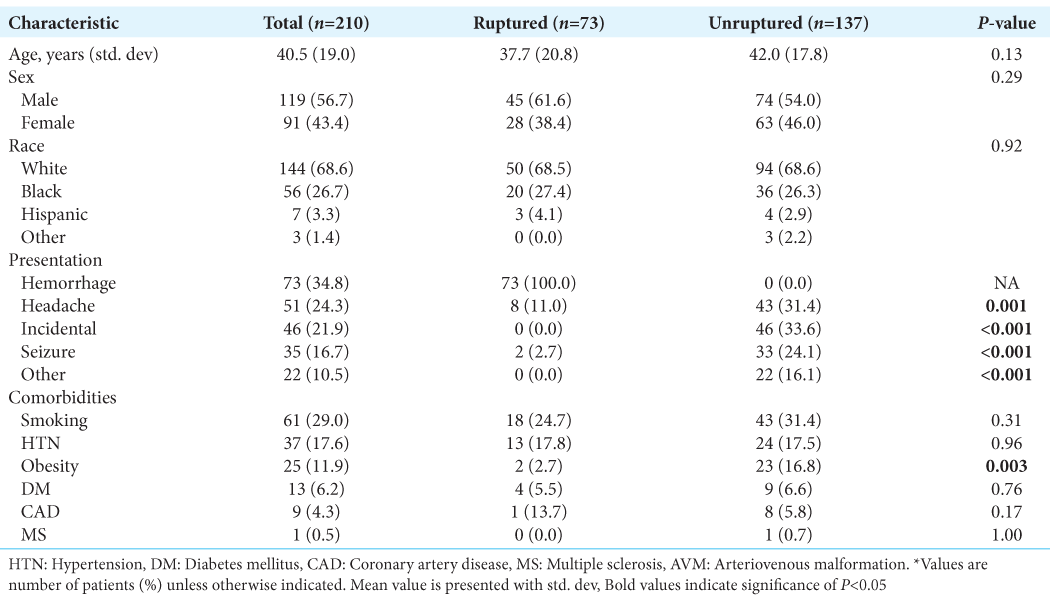
Of the 210 consecutive patients treated with SRS for intracranial AVMs, the average age was 41 years and 57% of patients were male. The most common AVM presentation was hemorrhage (35%), followed by headache (24%) and seizure (17%). The rate of incidentally discovered AVMs was 22%. Of the 210 patients, 73 presented with a ruptured AVM. [
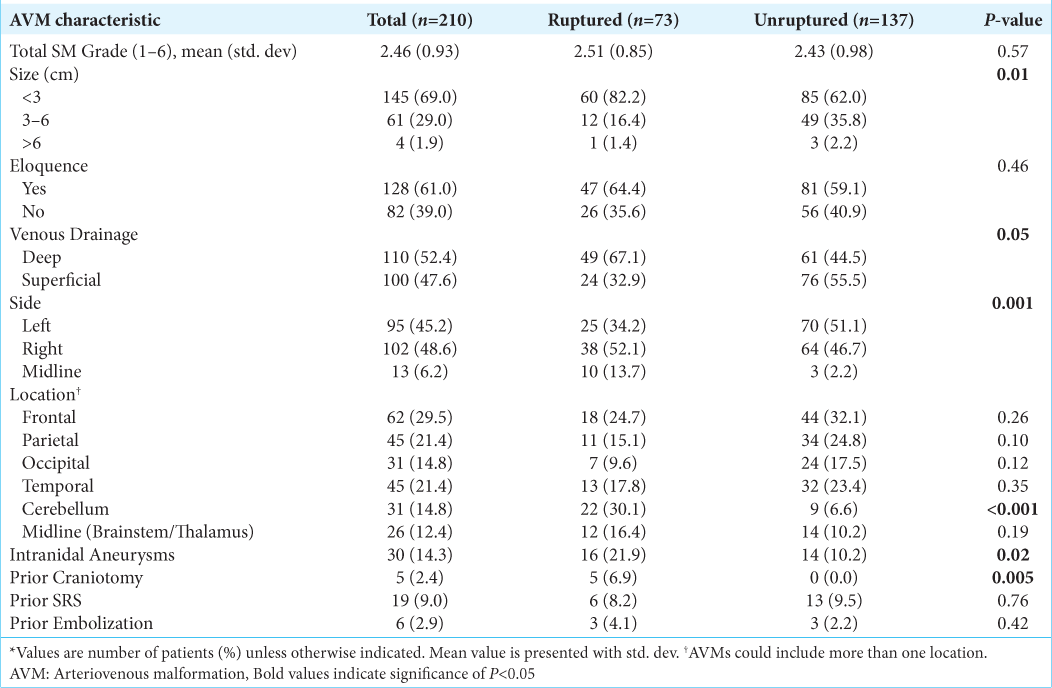
On binary logistic regression analysis, smaller AVM size (P = 0.01), deep venous drainage (P = 0.05), the presence of intranidal aneurysms (P = 0.02) as well as midline (P = 0.001), and cerebellar location (P < 0.001) were associated with AVM rupture at presentation. Prior craniotomy was significantly associated with ruptured AVM presentation (6.9% vs. 0%, P = 0.005) [
Post-SRS outcomes: Ruptured versus unruptured presentation
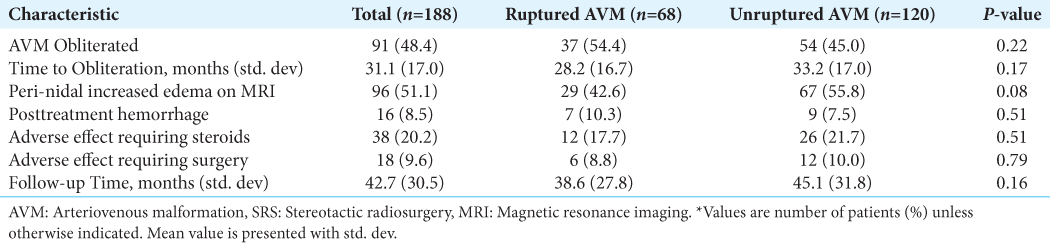
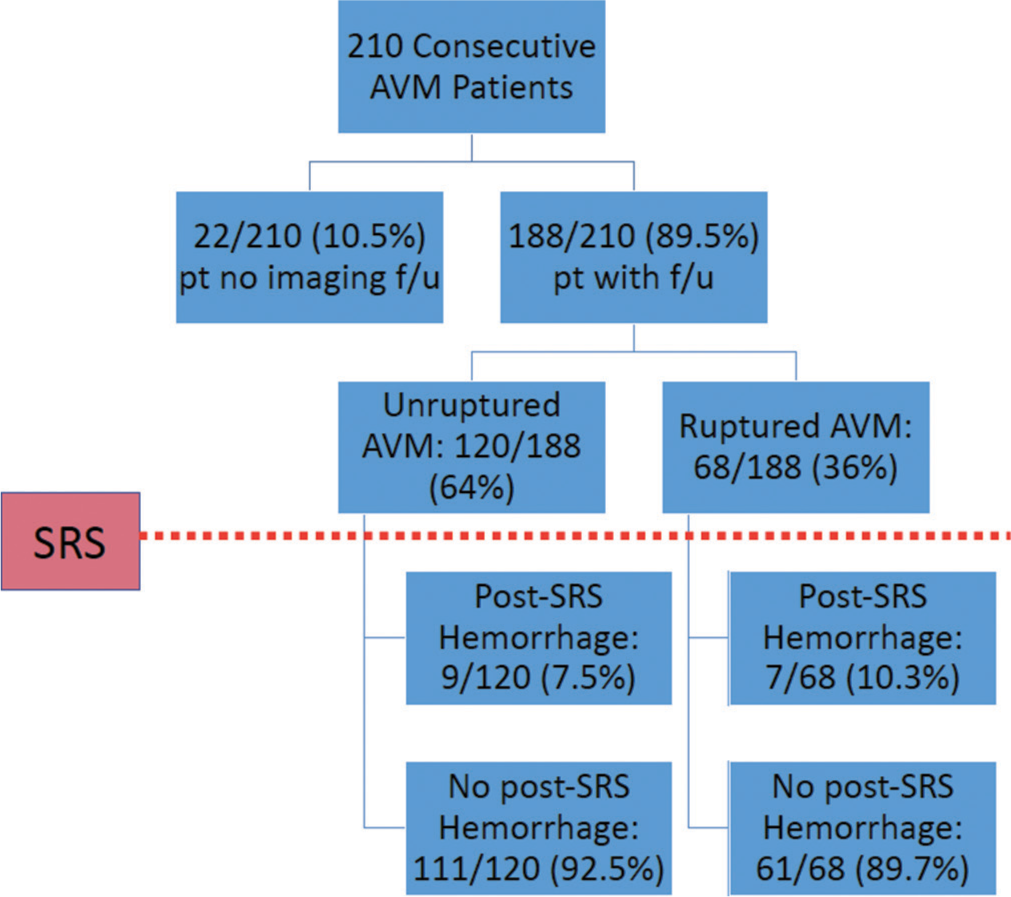
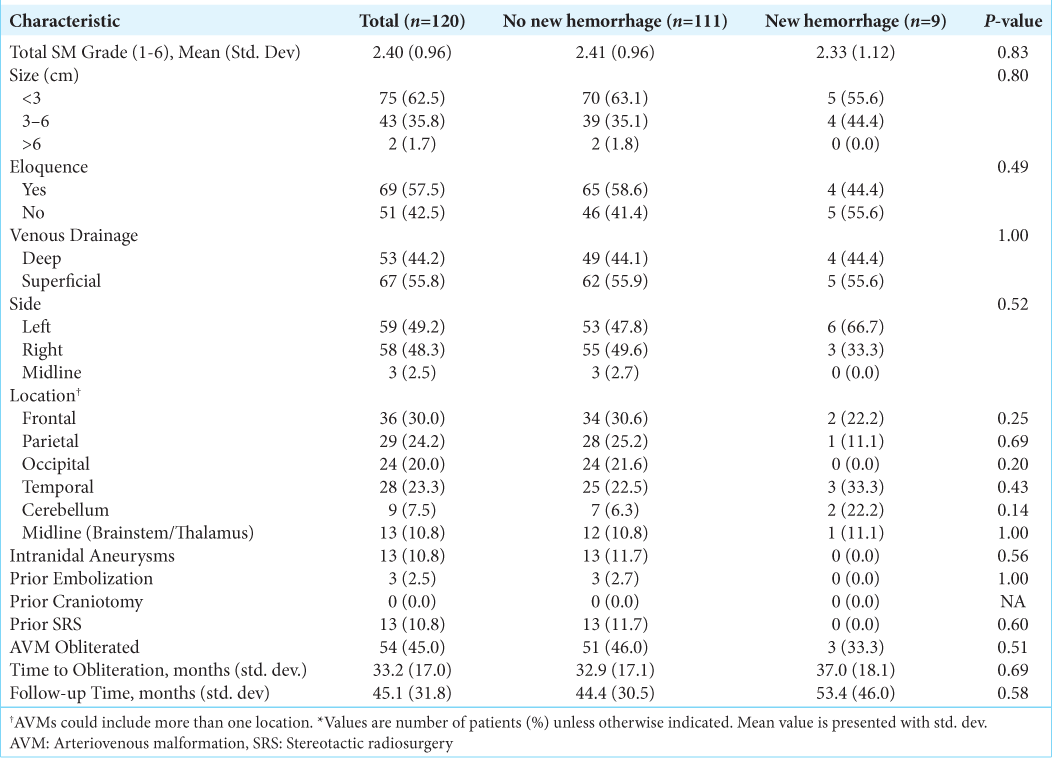
Overall, 188/210 patients (90%) had complete follow-up information available. Of these, 68 patients (36%) presented with a ruptured AVM and 120 (64%) were unruptured. The average follow-up time was 43 ± 31 months and was not significantly different between groups (P = 0.16). Overall obliteration rate was 48.4% and while a higher percentage of ruptured AVMs was obliterated (54.4 vs. 45%), this was not statistically significant (P = 0.22). Mean time to obliteration was also not significantly different between ruptured and unruptured groups (28 vs. 33 months, P = 0.17) [
Post-SRS complications: Ruptured versus unruptured presentation
Overall, there was a 20.2% rate of adverse effect requiring steroids, 9.6% rate of adverse effect requiring surgery, and 51.1% of patients developed increased peri-nidal T2 edema on post-operative MRI. None of these post-SRS complication rates were significantly different between ruptured and unruptured groups [
AVM factors associated with post-SRS hemorrhage: Ruptured and unruptured presentation
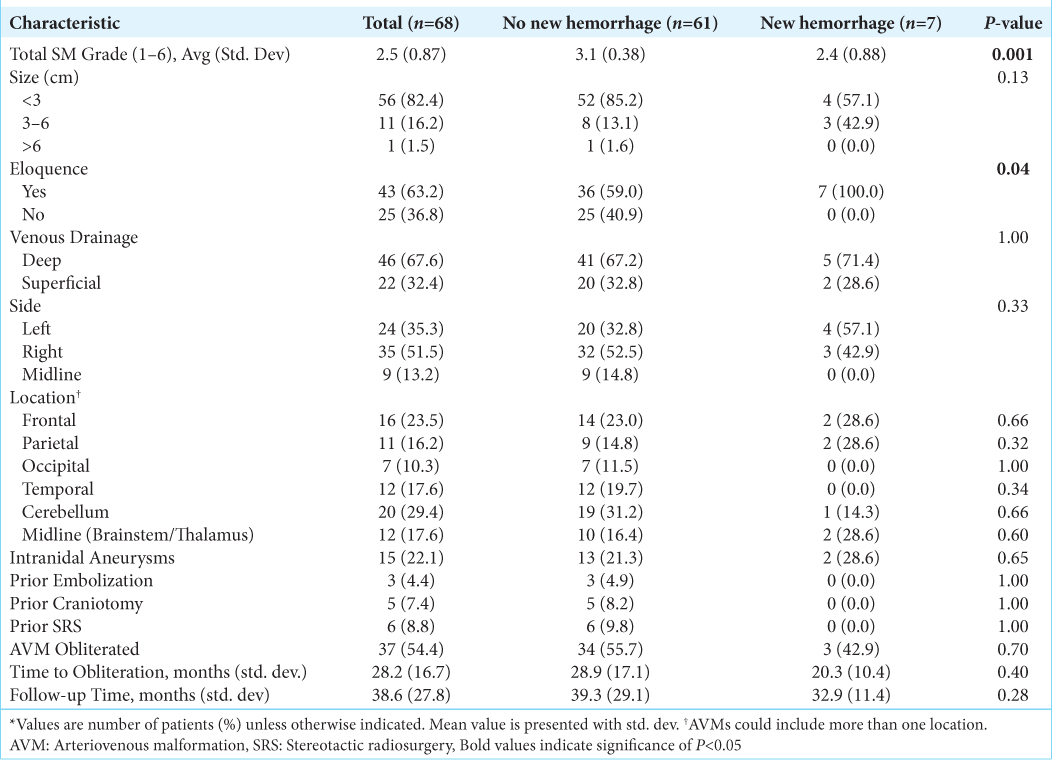
In patients presenting with ruptured AVMs, lower SM grade (P = 0.001) and eloquent AVM location (P = 0.04) were associated with the development of post-SRS hemorrhage [
DISCUSSION
ICH is the most common presentation of cerebral AVMs and results in significant morbidity and mortality.[
SRS remains an effective treatment option for AVMs with the goal of complete obliteration that typically occurs over a period of 6 months to up to 3 years.[
In the present study, hemorrhage was the most common presentation of AVMs and was associated with smaller AVM size, deep venous drainage, and the presence of intranidal aneurysms; findings consistent with much of the prior literature. In addition, cerebellar location was found to be significantly associated with ruptured presentation, consistent with prior literature demonstrating that cerebellar AVMs rarely present with seizures, most commonly present with hemorrhage, and have a more aggressive natural history.[
In an analysis of AVM hemorrhage risk before and after SRS, Ding et al. identified larger AVM size to be positively associated with and deep venous drainage to be negatively associated with hemorrhage before SRS.[
The present study found an overall post-SRS hemorrhage rate of 8.5% that was not significantly different between those presenting with ruptured and unruptured AVMs over a 43 month mean follow-up time. Development of a new hemorrhage after SRS in patients presenting with a ruptured AVM was associated with an overall lower SM score and eloquent AVM location; however, no factors were associated with new post-SRS hemorrhage in patients presenting with unruptured AVMs. In contrast to prior studies, the presence of intranidal aneurysms was not associated with an increased risk of new AVM hemorrhage after SRS in either group (ruptured vs. unruptured).[
While prior studies have examined outcomes after SRS in adults with hemorrhagic AVMs, few have compared the post-SRS complication rates of ruptured versus unruptured patient populations. Kawashima et al. reported a low post-SRS hemorrhage risk of 1.5% within 5 years and concluded that stand-alone SRS is effective for hemorrhagic AVMs.[
The present study reports a non-statistically significant increased rate of post-SRS hemorrhage in those with ruptured versus unruptured AVMs that are consistent with prior natural history reports. This suggests that SRS did not alter the natural risk of hemorrhage during the follow-up period in either group. This study additionally reports similar rates of obliteration, perinidal increases in T2 edema on MRI and adverse effects requiring steroids or surgery after SRS between those presenting with ruptured and unruptured AVMs suggesting that overall outcomes and complication profiles after SRS are not altered by a ruptured AVM presentation.
Limitations
This retrospective study is limited by its retrospective design, small number of patients presenting with hemorrhage and developing hemorrhage after SRS along with variability in follow-up. Retrospective studies are subject to unidentified confounders, imbalanced cohorts, incomplete data sets, misinterpretation of data, recall bias, selection bias, and observer bias. Specifically, the selection biases of a purely SRS single-center study limit the generalizability and reproducibility of the analysis and findings as patients undergoing SRS for ruptured AVMs are likely to have smaller AVMs in deeper locations that make them suboptimal candidates for open surgery. The small number of patients developing a post-SRS hemorrhages in each group also underpowered the ability to detect patient and AVM-related associations with new hemorrhage after SRS. In addition, the time to hemorrhage after SRS was not available, only the presence or absence, limiting the ability to develop conclusions regarding the temporal relationships between obliteration, and post-SRS hemorrhage development.
CONCLUSION
This study affirms findings from prior studies that smaller AVM size, deep venous drainage pattern, and associated intranidal aneurysms are associated with ruptured presentation. Rates of post-SRS recurrent hemorrhage, increased T2 edema, and adverse effects requiring steroids or surgery were similar between unruptured and ruptured AVM groups indicating a similar post-SRS risk profile in these patient populations. SRS did not appear to alter the natural history of recurrent AVM hemorrhage in this population.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Shahi R, Warlow C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain. 2001. 124: 1900-26
2. Arnaout OM, Gross BA, Eddleman CS, Bendok BR, Getch CC, Batjer HH. Posterior fossa arteriovenous malformations. Neurosurg Focus. 2009. 26: E12
3. Chen CJ, Ding D, Derdeyn CP, Lanzino G, Friedlander RM, Southerland AM. Brain arteriovenous malformations: A review of natural history, pathobiology, and interventions. Neurology. 2020. 95: 917-27
4. Chen CJ, Lee CC, Ding D, Tzeng SW, Kearns KN, Kano H. Stereotactic radiosurgery for unruptured versus ruptured pediatric brain arteriovenous malformations. Stroke. 2019. 50: 2745-51
5. Crawford PM, West CR, Chadwick DW, Shaw MD. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986. 49: 1-10
6. Ding D, Chen CJ, Starke RM, Kano H, Lee JY, Mathieu D. Risk of brain arteriovenous malformation hemorrhage before and after stereotactic radiosurgery. Stroke. 2019. 50: 1384-91
7. Ding D, Yen CP, Starke RM, Xu Z, Sheehan JP. Effect of prior hemorrhage on intracranial arteriovenous malformation radiosurgery outcomes. Cerebrovasc Dis. 2015. 39: 53-62
8. Drake CG. Cerebral arteriovenous malformations: Considerations for and experience with surgical treatment in 166 cases. Clin Neurosurg. 1979. 26: 145-208
9. Fults D, Kelly DL. Natural history of arteriovenous malformations of the brain: A clinical study. Neurosurgery. 1984. 15: 658-62
10. Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983. 58: 331-7
11. Hartmann A, Mast H, Mohr JP, Koennecke HC, Osipov A, Pile-Spellman J. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998. 29: 931-4
12. Ilyas A, Chen CJ, Ding D, Buell TJ, Raper DM, Lee CC. Radiation-induced changes after stereotactic radiosurgery for brain arteriovenous malformations: A systematic review and meta-analysis. Neurosurgery. 2018. 83: 365-76
13. Ilyas A, Chen CJ, Ding D, Mastorakos P, Taylor DG, Pomeraniec IJ. Cyst formation after stereotactic radiosurgery for brain arteriovenous malformations: A systematic review. J Neurosurg. 2018. 128: 1354-63
14. Jayaraman MV, Marcellus ML, Do HM, Chang SD, Rosenberg JK, Steinberg GK. Hemorrhage rate in patients with Spetzler-Martin grades IV and V arteriovenous malformations: Is treatment justified?. Stroke. 2007. 38: 325-9
15. Kano H, Flickinger JC, Tonetti D, Hsu A, Yang HC, Flannery TJ. Estimating the risks of adverse radiation effects after gamma knife radiosurgery for arteriovenous malformations. Stroke. 2017. 48: 84-90
16. Kano H, Kondziolka D, Flickinger JC, Yang HC, Park KJ, Flannery TJ. Aneurysms increase the risk of rebleeding after stereotactic radiosurgery for hemorrhagic arteriovenous malformations. Stroke. 2012. 43: 2586-91
17. Karlsson B, Lax I, Söderman M. Risk for hemorrhage during the 2-year latency period following gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2001. 49: 1045-51
18. Kawashima M, Hasegawa H, Shin M, Shinya Y, Ishikawa O, Koizumi S. Outcomes of stereotactic radiosurgery for hemorrhagic arteriovenous malformations with or without prior resection or embolization. J Neurosurg. 2020. p.
19. Khaw AV, Mohr JP, Sciacca RR, Schumacher HC, Hartmann A, Pile-Spellman J. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke. 2004. 35: 660-3
20. Kim H, Al-Shahi Salman R, McCulloch CE, Stapf C, Young WL. Untreated brain arteriovenous malformation: Patient-level meta-analysis of hemorrhage predictors. Neurology. 2014. 83: 590-7
21. Kondziolka D, McLaughlin MR, Kestle JR. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 1995. 37: 851-5
22. Laakso A, Dashti R, Juvela S, Isarakul P, Niemelä M, Hernesniemi J. Risk of hemorrhage in patients with untreated Spetzler-Martin grade IV and V arteriovenous malformations: A long-term follow-up study in 63 patients. Neurosurgery. 2011. 68: 372-7
23. Laakso A, Dashti R, Seppänen J, Juvela S, Väärt K, Niemelä M. Long-term excess mortality in 623 patients with brain arteriovenous malformations. Neurosurgery. 2008. 63: 244-53
24. Maruyama K, Kawahara N, Shin M, Tago M, Kishimoto J, Kurita H. The risk of hemorrhage after radiosurgery for cerebral arteriovenous malformations. N Engl J Med. 2005. 352: 146-53
25. Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: A 24-year follow-up assessment. J Neurosurg. 1990. 73: 387-91
26. Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg. 1966. 25: 467-90
27. Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ, Kondziolka D. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke. 1996. 27: 1-6
28. Pollock BE, Flickinger JC, Lunsford LD, Maitz A, Kondziolka D. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurg. 1998. 42: 1239-44
29. Pomeraniec IJ, Ding D, Starke RM, Liu KC, Mrachek EK, Lopes MB. Delayed cyst formation after stereotactic radiosurgery for brain arteriovenous malformations. J Neurosurg. 2018. 129: 937-46
30. Schneider BF, Eberhard DA, Steiner LE. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997. 87: 352-7
31. Shuto T, Ohtake M, Matsunaga S. Proposed mechanism for cyst formation and enlargement following gamma knife surgery for arteriovenous malformations. J Neurosurg. 2012. 117: 135-43
32. Spetzler RF, Hargraves RW, McCormick PW, Zabramski JM, Flom RA, Zimmerman RS. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992. 76: 918-23
33. Wilkins RH. Natural history of intracranial vascular malformations: A review. Neurosurgery. 1985. 16: 421-30