- Department of Neurosurgery, The University of Tokyo Hospital, Tokyo, Japan,
- Department of Neurosurgery, Teikyo University Hospital, Tokyo, Japan,
- Department of Radiology, The University of Tokyo Hospital, Tokyo, Japan,
- Department of Pathology, The University of Tokyo Hospital, Tokyo, Japan,
- Department of Otorhinolaryngology, The University of Tokyo Hospital, Tokyo, Japan.
Correspondence Address:
Motoyuki Umekawa, Department of Neurosurgery, The University of Tokyo Hospital, Tokyo, Japan.
DOI:10.25259/SNI_675_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yuki Nakamura1, Motoyuki Umekawa1, Yuki Shinya1, Hirotaka Hasegawa1, Masahiro Shin2, Atsuto Katano3, Aya Shinozaki-Ushiku4, Kenji Kondo5, Nobuhito Saito1. Stereotactic radiosurgery for skull base adenoid cystic carcinoma: A report of two cases. 04-Nov-2022;13:509
How to cite this URL: Yuki Nakamura1, Motoyuki Umekawa1, Yuki Shinya1, Hirotaka Hasegawa1, Masahiro Shin2, Atsuto Katano3, Aya Shinozaki-Ushiku4, Kenji Kondo5, Nobuhito Saito1. Stereotactic radiosurgery for skull base adenoid cystic carcinoma: A report of two cases. 04-Nov-2022;13:509. Available from: https://surgicalneurologyint.com/surgicalint-articles/11976/
Abstract
Background: Adenoid cystic carcinoma (ACC) is an uncommon salivary gland tumor with a relatively favorable prognosis. However, treating ACC is potentially challenging because radical resection is usually difficult once the skull base is involved due to the adjacent critical structures. Stereotactic radiosurgery (SRS) is a less invasive alternative for surgically recalcitrant lesions.
Case Description: We report two patients with three metastatic skull base ACCs who underwent SRS using the Gamma Knife with a marginal dose of 20 Gy to a 50% isodose line. All tumors were effectively controlled without any adverse events.
Conclusion: This case report and our review of the literature suggest that SRS can be considered for local control of ACC invading the skull base when surgical resection is unsuitable or a postoperative residual lesion is suspected. Further, investigations on the accumulated subjects are warranted to confirm the role of SRS for the treatment of ACCs.
Keywords: Adenoid cystic carcinoma, Case report, Recurrence, Skull base, Stereotactic radiosurgery
INTRODUCTION
Adenoid cystic carcinoma (ACC) accounts for only ~1% of head and neck malignancies; it typically arises from salivary glands.[
Stereotactic radiosurgery (SRS) comprises focused high-dose irradiation at a single session. It yields 1-year local control of 60–90% of malignant metastatic brain tumors and is efficacious in the treatment of brain metastases and skull base tumors.[
CASE PRESENTATIONS
Case 1
A 50-year-old man with a nosebleed was referred to our hospital. He had a medical history of pituitary adenoma which was totally resected at the age of 17 without recurrence, myocardial infarction, hypertension, and dyslipidemia. Magnetic resonance imaging (MRI) revealed a heterogeneously enhanced tumor extending from the nasal cavity to the suprasellar region [
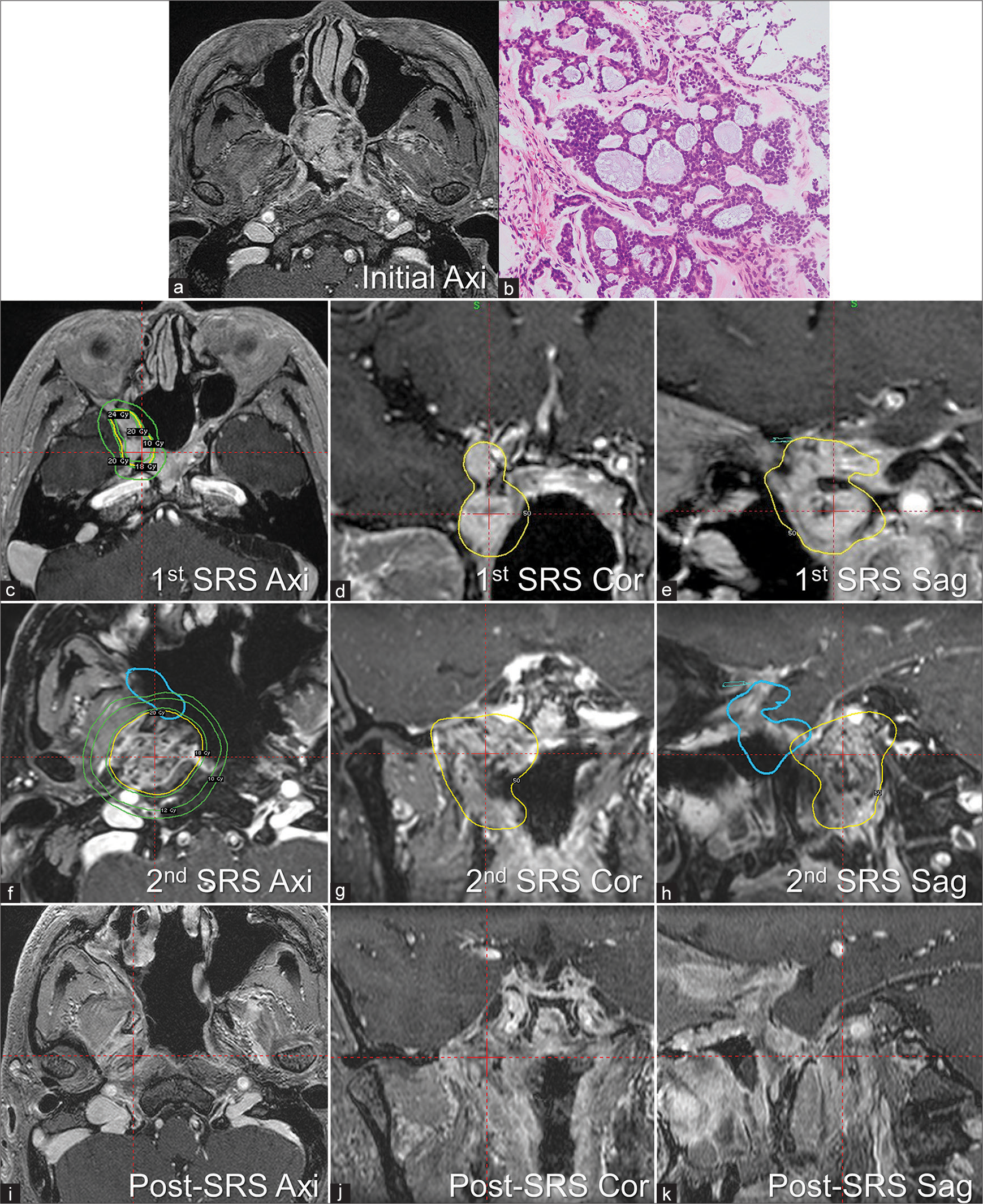
Figure 1:
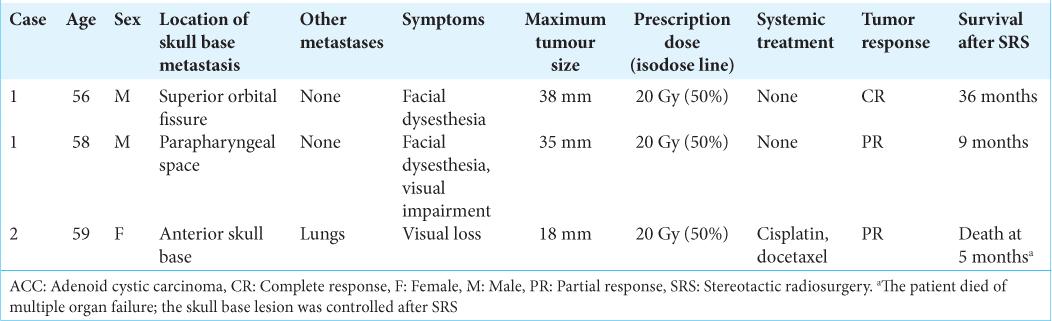
Adenoid cystic carcinoma (ACC) in a 50-year-old man (Case 1). Gadolinium-enhanced, T1-weighted magnetic resonance, imaging revealed an enhanced tumor extending from the nasal cavity to the suprasellar region (a). The tumor was pathologically confirmed as ACC, exhibiting cribriform growth with myoepithelial differentiation (b). After two resections, he experienced recurrence in the superior orbital fissure, at age 56, and stereotactic radiosurgery (SRS) was performed with a marginal dose of 20 Gy to a 50% isodose line (yellow line) in axial (c), coronal (d), and sagittal plane (e) Green lines indicated marginal dose line of 10 Gy, 18 Gy, and 24 Gy. A remote metastasis was detected in the parapharyngeal space, with marginal recurrence after its resection. We performed the second SRS for the parapharyngeal metastasis with the same parameters and the planning was shown in axial (f), coronal (g), and sagittal plane (h) with the previous planning in blue line. Yellow line indicated 20 Gy to a 50% isodose line, and green lines indicated marginal dose line of 10 Gy, 12 Gy, and 18 Gy. Both lesions were in remission at the last follow-up: 36 months after the first SRS for recurrence in the superior orbital fissure and 9 months after the second SRS for recurrence in the parapharyngeal space (i-k).
Case 2
A 56-year-old woman with the right facial numbness and trigeminal neuralgia was referred to our hospital. She had a medical history of gastric cancer which was treated with surgery 7 years before her visit and showed no recurrence. MRI revealed a large tumor extending from the orbit to the cavernous sinus. We considered this skull base lesion as the primary site, because the initial whole body-CT scan revealed no evidence of any tumor. It was resected and pathologically confirmed as ACC arising from the lacrimal gland [
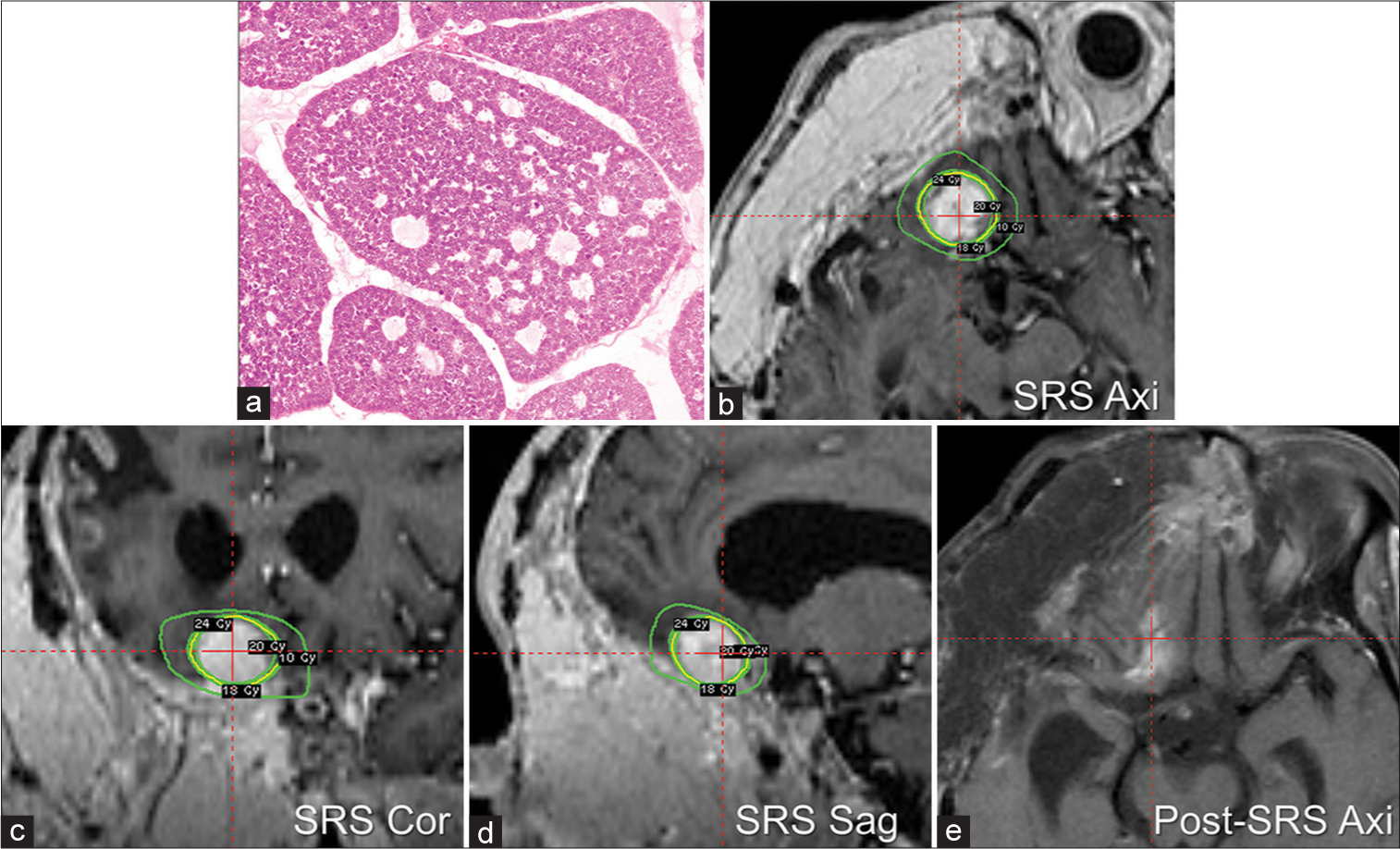
Figure 2:
A 56-year-old woman underwent resection for a tumor growing next to the right orbit and the anterior skull base (Case 2). The tumor was pathologically diagnosed as adenoid cystic carcinoma arising from the lacrimal gland (a). Despite repeated resection, she experienced recurrence around the right cavernous sinus and orbit. We performed stereotactic radiosurgery for the anterior skull base metastasis, with a marginal dose of 20 Gy to a 50% isodose line (yellow line) in axial (b), coronal (c), and sagittal plane (d) Green lines indicated marginal dose line of 10 Gy, 18 Gy, and 24 Gy. The tumor shrank considerably in the next 5 months (e).
DISCUSSION
We reported on two cases of skull base metastatic ACCs that were successfully controlled with SRS without significant adverse radiation effects. Patients with ACCs have a relatively favorable prognosis even with metastatic tumors; the 5-, 10-, and 15-year survival rates for all stages of ACCs are 90%, 80%, and 69%, respectively.[
The minimally invasive nature and low rate of treatment-associated complications of SRS can be a major benefit for ACC, in which frequent local recurrence is a major issue. Although surgical removal is the standard treatment for the primary tumors, the optimal strategy for recurrent ACC remains to be determined. Given the possibility of various perioperative complications,[
CONCLUSION
We described two cases with three skull base metastases of ACC, for which we achieved successful local control with Gamma Knife-based SRS. SRS is a promising treatment option for skull base ACCs, both in terms of local control and functional preservation. Further, observations in more cases are required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
JSPS KAKENHI (grant number: 20K17919).
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Atallah S, Marc M, Schernberg A, Huguet F, Wagner I, Makitie A. Beyond surgical treatment in adenoid cystic carcinoma of the head and neck: A literature review. Cancer Manag Res. 2022. 14: 1879-90
2. Cantu G. Adenoid cystic carcinoma. An indolent but aggressive tumour Part B: Treatment and prognosis. Acta Otorhinolaryngol Ital. 2021. 41: 296-307
3. Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL. Adenoid cystic carcinoma of the head and neck--An update. Oral Oncol. 2015. 51: 652-61
4. Cohen AN, Damrose EJ, Huang RY, Nelson SD, Blackwell KE, Calcaterra TC. Adenoid cystic carcinoma of the submandibular gland: A 35-year review. Otolaryngol Head Neck Surg. 2004. 131: 994-1000
5. Dias FL, Sa GM, Kligerman J, Lopes HF, Wance JR, Paiva FP. Complications of anterior craniofacial resection. Head Neck. 1999. 21: 12-20
6. Diaz EM, Kies MS. Chemotherapy for skull base cancers. Otolaryngol Clin North Am. 2001. 34: 1079-85
7. Ellington CL, Goodman M, Kono SA, Grist W, Wadsworth T, Chen AY. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973-2007 surveillance, epidemiology, and end results data. Cancer. 2012. 118: 4444-51
8. Harris S, Chan MD, Lovato JF, Ellis TL, Tatter SB, Bourland JD. Gamma knife stereotactic radiosurgery as salvage therapy after failure of whole-brain radiotherapy in patients with small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012. 83: e53-9
9. Koka VN, Tiwari RM, van der Waal I, Snow GB, Nauta J, Karim AB. Adenoid cystic carcinoma of the salivary glands: Clinicopathological survey of 51 patients. J Laryngol Otol. 1989. 103: 675-9
10. Kryzanski JT, Annino DJ, Gopal H, Heilman CB. Low complication rates of cranial and craniofacial approaches to midline anterior skull base lesions. Skull Base. 2008. 18: 229-41
11. Lorini L, Ardighieri L, Bozzola A, Romani C, Bignotti E, Buglione M. Prognosis and management of recurrent and/or metastatic head and neck adenoid cystic carcinoma. Oral Oncol. 2021. 115: 105213
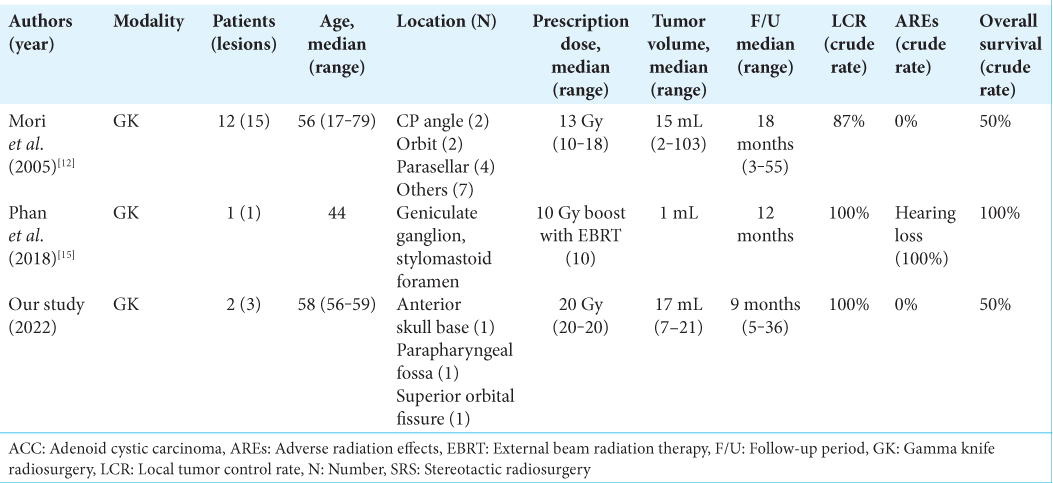
12. Mori Y, Kobayashi T, Kida Y, Oda K, Shibamoto Y, Yoshida J. Stereotactic radiosurgery as a salvage treatment for recurrent skull base adenoid cystic carcinoma. Stereotact Funct Neurosurg. 2005. 83: 202-7
13. Naunheim MR, Sedaghat AR, Lin DT, Bleier BS, Holbrook EH, Curry WT. Immediate and delayed complications following endoscopic skull base surgery. J Neurol Surg B Skull Base. 2015. 76: 390-6
14. Nieder C, Grosu AL, Gaspar LE. Stereotactic radiosurgery (SRS) for brain metastases: A systematic review. Radiat Oncol. 2014. 9: 155
15. Phan JL, Pollard C, Wang H, Ng SP, Sheu T, Ginsberg LE. Dosimetric advantages of stereotactic radiosurgery as a boost to adjuvant conventional radiotherapy in the setting of adenoid cystic carcinoma of the parotid with skull base invasion. Clin Case Rep. 2018. 6: 2126-30
16. Sahara S, Herzog AE, Nor JE. Systemic therapies for salivary gland adenoid cystic carcinoma. Am J Cancer Res. 2021. 11: 4092-110
17. Shigematsu H, Magoshi S, Suzuki S, Kusama K, Sakashita H. An apparent radiation-induced carcinoma of the parotid gland following treatment for adenoid cystic carcinoma of the sublingual gland: A case report. J Oral Maxillofac Surg. 2004. 62: 1169-74
18. Sokoya M, Mourad M, Ducic Y. Complications of skull base surgery. Semin Plast Surg. 2017. 31: 227-30
19. Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D. Treatment for brain metastases: ASCOSNO-ASTRO guideline. J Clin Oncol. 2022. 40: 492-516
20. Wakisaka S, Nonaka A, Morita Y, Fukui M, Kinoshita K. Adenoid cystic carcinoma with intracranial extension: Report of three cases. Neurosurgery. 1990. 26: 1060-5
21. Wang L, Jia T, Zhao R, Zhu L, Zhang H. I 125 brachytherapy for central adenoid cystic carcinoma of the mandible. Oral and Maxillofac Surg Cases. 2018. 4: 53-7
22. Webb PS, Zhang YZ, Burrell K, Sinclair G. Adenoid cystic carcinoma and chronic lymphocytic leukaemia: Synchronous presentations in the lung. BMJ Case Rep. 2021. 14: e236074
23. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014. 15: 387-95