- Department of Neurosurgery, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India.
DOI:10.25259/SNI_756_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: G. Lakshmi Prasad. Steroids for delayed cerebral edema after traumatic brain injury. 10-Feb-2021;12:46
How to cite this URL: G. Lakshmi Prasad. Steroids for delayed cerebral edema after traumatic brain injury. 10-Feb-2021;12:46. Available from: https://surgicalneurologyint.com/surgicalint-articles/10586/
Abstract
Background: Brain edema is a common phenomenon after traumatic brain injury (TBI) resulting in increased intracranial pressure and subsequent neurological deterioration. Experimental studies have proven that brain edema is biphasic (cytotoxic followed by vasogenic). Till date, all studies, including the corticosteroid randomization after significant head injury (HI) trial, have used high-dose steroids in the acute period during which the edema is essentially cytotoxic in nature. No clinical data exist pertaining to delayed cerebral edema (vasogenic) and steroids.
Methods: Patients who had received steroids for delayed cerebral edema after TBI were retrospectively analyzed over a 2-year period. Steroid dose, timing of steroid prescription, time to improvement of symptoms, and complications were noted.
Results: There were six males and three females. Mean age was 41.1 years. There were no severe HI cases. All subjects had cerebral contusions on imaging. Dexamethasone was the preferred steroid starting with 12 mg/day and tapered in 5–7 days. The mean interval to steroid administration after trauma was 7 days. The mean duration of steroid prescription was 6.3 days. All patients had complete symptomatic improvement. The mean time to symptom resolution was 3.8 days. No patients experienced any complications pertinent to steroid usage.
Conclusion: This is the first study to document efficacy of steroids for delayed cerebral edema after TBI, at least in mild/moderate head injuries. The timing of steroid usage and dose of steroids is key aspects that might determine its efficacy in TBI which was the drawbacks of the previous studies. Future prospective trials with the above factors in consideration may confirm/refute above findings.
Keywords: Brain edema, Corticosteroids, Delayed cerebral edema, Steroids, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is one of the most common causes of morbidity and mortality.[
MATERIALS AND METHODS
Patients who received steroids for delayed cerebral edema after TBI were retrospectively analyzed over a 2-year period. Hospital records were utilized for retrieval of data. Variables analyzed included age, gender, mode of injury, nature of symptoms (headache, vomiting, focal neurological deficits, and neurological deterioration), admission Glasgow coma scale (GCS) score, pupillary reactivity, computed tomography (CT) findings (contusion, subdural hematoma [SDH], diffuse axonal injury, cerebral edema, status of basal cisterns, midline shift [MLS] etc.), time to clinical deterioration after trauma (either a drop in GCS score or worsening of symptoms), dosage of steroids, duration of steroid treatment, time to clinical and radiological improvement, and Glasgow Outcome Scale score at discharge at follow-up and follow-up duration.
Steroids were prescribed only in those cases where the cerebral edema persisted and patient showed worsening/ non-improvement of symptoms despite administration of standard cerebral decongestants such as mannitol/ hypertonic saline. Dexamethasone was used in all subjects and was administered parenterally for 24–48 h that was later converted to oral tapering doses for a total duration of 5–7 days.
RESULTS
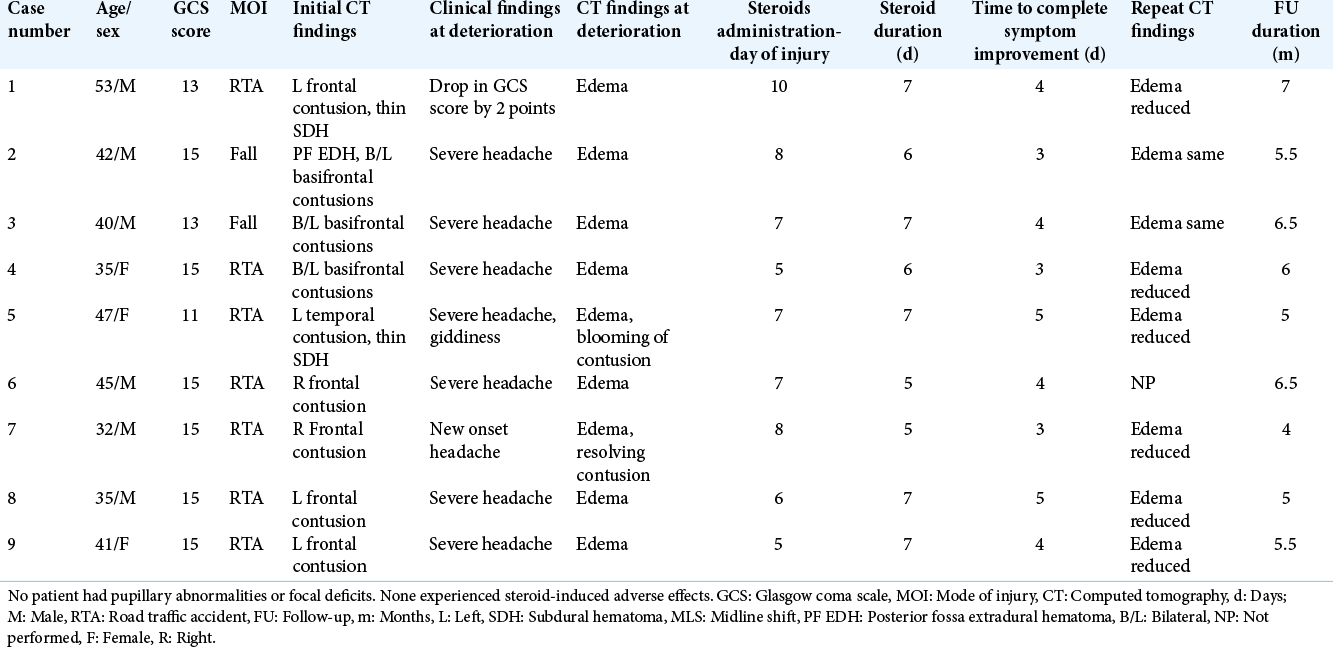
Of the nine cases, there were six males and three females. The mean age of the cohort was 41.1 years (SD = ±6.62). Six subjects sustained TBI as a result of road traffic accidents (RTA). Based on GCS scores, there were eight mild and one moderate head injuries (mainly because of low verbal output). The median admission GCS score was 15. The mean GCS score was 13.4 (SD = ±2.60). No patients had focal neurological deficits or pupillary abnormalities on admission.
On radiology, all subjects had parenchymal contusions (five unifrontal, three bifrontal, and one temporal), two subjects had additional thin acute SDH and one subject had additional posterior fossa extradural hematoma. Basal cisterns were partially effaced in seven cases. MLS was noted in four cases and the mean MLS among them was 4.7 mm (SD = ±1.25). CT scan done at the time of clinical worsening showed increased edema in all cases with additional blooming of contusion in one case.
The patient of moderate HI had a drop in GCS score by two points, while other cases developed worsening of headache/ new-onset disabling headache. The mean time to steroid administration after the trauma was 7 days (SD = ±1.58). The mean duration of steroid prescription was 6.3 days (SD = ±0.86). All patients had symptomatic improvement and the mean time to symptom resolution was 3.8 days (SD = ±0.78). Repeat CT after steroids was performed in eight cases. There was no change in cerebral edema in two cases, while reduction of cerebral edema was seen in six cases. The median GCS score at discharge was 15. None of the subjects suffered any complications attributable to the steroid usage. The mean follow-up duration was 5.6 months (SD = ±0.93).
Representative case description
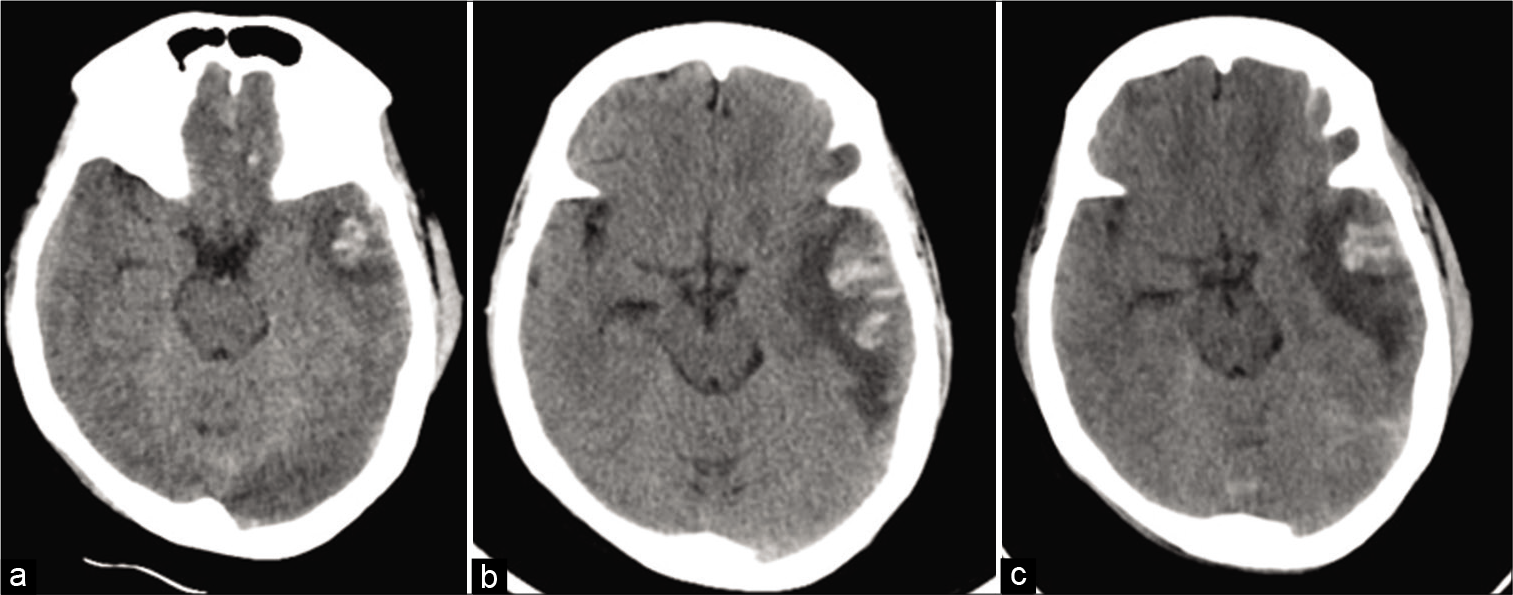
Case 5: A 47-year female presented to our emergency services after an alleged RTA 1 day prior. She complained of mild-to-moderate left-sided headache, not associated with vomiting. On examination, she was conscious, alert with coherent speech, and bilaterally reactive pupils. CT scan showed small left temporal contusion with minimal mass effect [
Figure 1:
(a) Non-contrast computed tomography (CT) showing small left temporal contusion. (b) CT performed after the onset of disabling headache showing blooming of contusion and increase in the perilesional edema with partial effacement of basal cisterns. (c) CT done 5 days after steroid administration showing resolution of cerebral edema.
[
DISCUSSION
Although termed to be highly simplistic, brain edema is said to be of two major types – vasogenic and cytotoxic. Other subtypes include osmotic and interstitial edema.[
It has been proven in experimental studies that brain edema is actually biphasic in nature, with an initial cytotoxic component and a subsequent vasogenic one. This is because increases in cranial water content can only be derived from the vasculature.[
The role of steroids in clinical studies of TBI has been conceptualized from spinal cord injury trials, most notably the NASCIS, wherein the investigators found difference in outcomes with high-dose methylprednisolone, if given within 8 h of the spinal injury.[
Merits of the study
First clinical study to document the effectiveness of steroids for delayed cerebral edema after TBI that may be of potential benefit to patients, in whom standard decongestants have not benefitted. Furthermore, decompressive surgeries for such delayed brain edema may potentially be avoided. This may form a basis for future multicentric clinical trials.
Drawbacks
The study being retrospective in nature and the limited number of cases are the main drawbacks of the study.
CONCLUSION
This is the first study to document the efficacy of steroids for delayed cerebral edema after TBI and only in mild/moderate HI cases. All the nine patients noticed clinical improvement and none suffered any complications related to steroid usage. The timing of steroid usage and dose of steroids are important key aspects that might determine its efficacy in TBI. With these factors taken into consideration, future prospective trials with strict inclusion/exclusion criteria may confirm/refute the findings of this study and the possible role of steroids in delayed cerebral edema.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury.Results of the third national acute spinal cord injury randomized controlled trial. National acute spinal cord injury study. JAMA. 1997. 277: 1597-604
2. Chen G, Shi JX, Hang CH, Xie W, Liu J, Liu X. Inhibitory effect on cerebral inflammatory agents that accompany traumatic brain injury in a rat model: A potential neuroprotective mechanism of recombinant human erythropoietin (rhEPO). Neurosci Lett. 2007. 425: 177-82
3. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011. 335: 2-13
4. Dearden NM, Gibson JS, Gibson RM, Cameron MM. Effect of high-dose dexamethasone on outcome from severe head injury. J Neurosurg. 1986. 64: 81-8
5. Donkin JJ, Vink R. Mechanisms of cerebral edema in traumatic brain injury: Therapeutic developments. Curr Opin Neurol. 2010. 23: 293-9
6. Gabrielian L, Helps SC, Thornton E, Turner RJ, Leonard AV, Vink R. Substance P antagonists as a novel intervention for brain edema and raised intracranial pressure. Acta Neurochir Suppl. 2013. 118: 201-4
7. Hellal F, Bonnefont-Rousselot D, Croci N, Palmier B, Plotkine M, Marchand-Verrecchia C. Pattern of cerebral edema and hemorrhage in a mice model of diffuse brain injury. Neurosci Lett. 2004. 357: 21-4
8. Hue CD, Cho FS, Cao S, Bass CR, Meaney DF, Morrision B 3rd. Dexamethasone potentiates in vitro blood-brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J Cereb Blood Flow Metab. 2015. 35: 1191-8
9. Klatzo I. Presidental address.Neuropathological aspects of brain edema. J Neuropathol Exp Neurol. 1967. 26: 1-14
10. Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007. 22: E1
11. Nag S, Manias JL, Stewart DJ. Pathology and new players in the pathogenesis of brain edema. Acta Neuropathol. 2009. 118: 197-217
12. Nimmo AJ, Cernak I, Heath DL, Hu X, Bennett CJ, Vink R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides. 2004. 38: 40-7
13. Prasad GL, Anmol N, Menon GR. Outcome of traumatic brain injury in the elderly population: A tertiary center experience in a developing country. World Neurosurg. 2018. 111: e228-34
14. Prasad GL, Gupta DK, Mahapatra AK, Sharma BS. Surgical results of decompressive craniectomy in very young children: A level one trauma centre experience from India. Brain Inj. 2015. 29: 1717-24
15. Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): Randomised placebo-controlled trial. Lancet. 2004. 364: 1321-8
16. Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl. 2006. 96: 130-3
17. Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004. 129: 1021-9