- Department of Neurosurgery, Medical University of South Carolina, Charleston, South Carolina, United States.
- Department of Radiology, Wake Forest University, Winston-Salem, North Carolina, United States.
- Department of Neurosurgery, H. Lee Moffitt Cancer Center, Tampa, Florida, United States.
- Department of Clinical Neurophysiology, Medical University of South Carolina, Charleston, South Carolina, United States.
Correspondence Address:
Mohammed Abdul Alshareef
Department of Neurosurgery, Medical University of South Carolina, Charleston, South Carolina, United States.
DOI:10.25259/SNI_815_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammed Abdul Alshareef1, Gibson Klapthor2, Stephen R. Lowe3, Jessica Barley4, David Cachia1, Bruce M. Frankel1. Strategies for posterior-only minimally invasive surgery in thoracolumbar metastatic epidural spinal cord compression. 22-Dec-2020;11:462
How to cite this URL: Mohammed Abdul Alshareef1, Gibson Klapthor2, Stephen R. Lowe3, Jessica Barley4, David Cachia1, Bruce M. Frankel1. Strategies for posterior-only minimally invasive surgery in thoracolumbar metastatic epidural spinal cord compression. 22-Dec-2020;11:462. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10487
Abstract
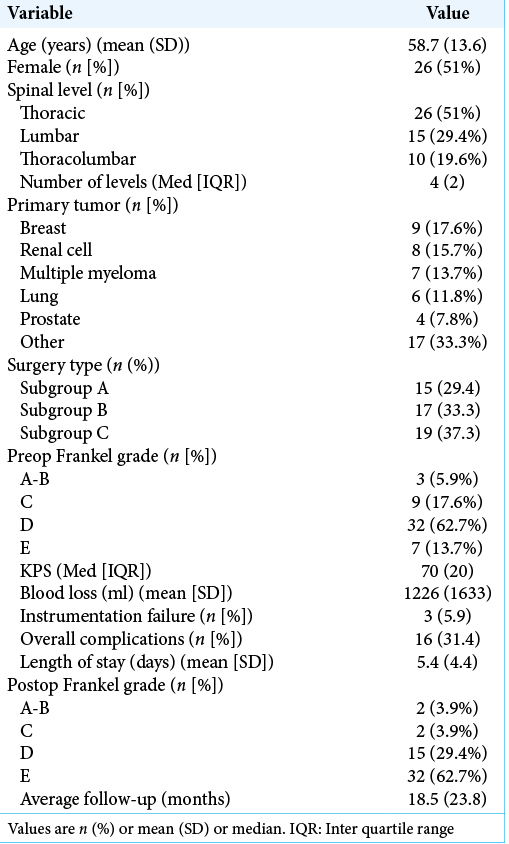
Background: Metastatic epidural spinal cord compression (MESCC) is a debilitating sequela of cancer. Here, we evaluated various subtypes of posterior-only minimally invasive spinal (MIS) procedures utilized to address different cancers.
Methods: Within this retrospective review, we analyzed the treatment of thoracolumbar MESCC treated with three MIS techniques: decompression and fusion (Subgroup A), partial corpectomy (Subgroup B), and full corpectomy (Subgroup C).
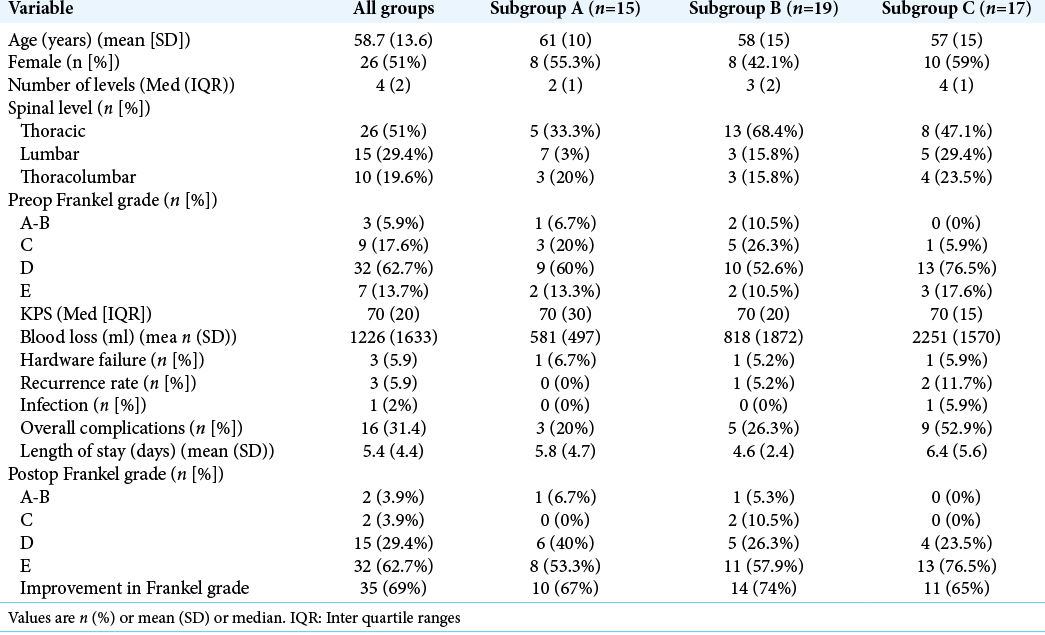
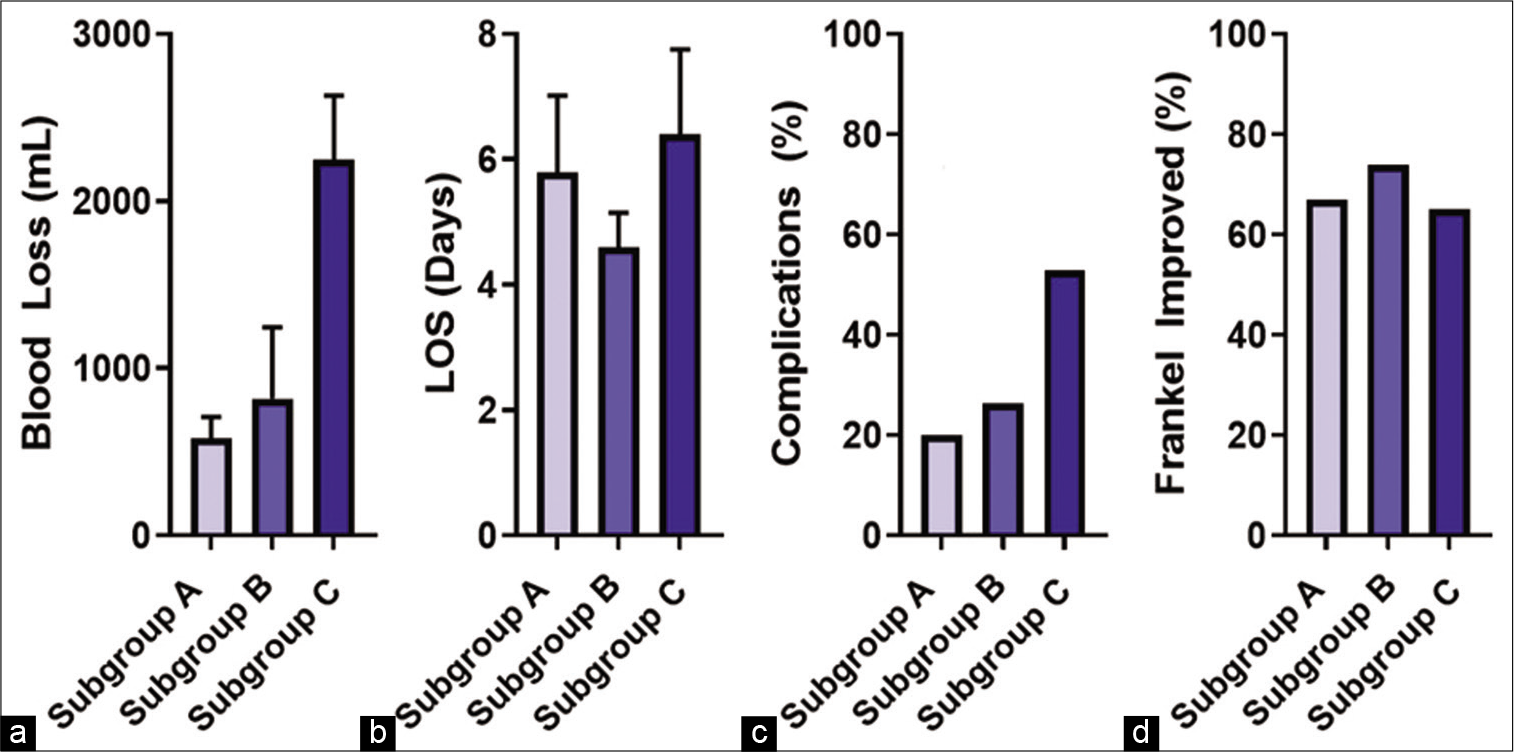
Results: There were 51 patients included in the study; they averaged 58.7 years of age, and 51% were females. Most tumors were in the thoracic spine (51%). The average preoperative Frankel grade was D (62.7%); 69% (35) improved postoperatively. The patients were divided as follows: subgroup A (15 patients = 29.4%), B (19 patients = 37.3%), and C (17 patients = 33.3%). The length of hospitalization was similar (~5.4 days) for all groups. The overall complication rate was 31%, while blood loss was lower in Subgroups A and B versus C.
Conclusion: Different MIS surgical techniques were utilized in patients with thoracic and/or lumbar MESCC. Interestingly, clinical outcomes were similar between MIS subgroups, in this study, with a trend toward higher complications and greater blood loss associated with those undergoing more aggressive MIS procedures (e.g., full corpectomy and fusion).
Keywords: Corpectomy, Metastasis, Metastatic epidural spinal cord compression, Minimally invasive spine, Spine surgery, Thoracolumbar
INTRODUCTION
The thoracolumbar spine is the most common osseous site for malignant neoplasm metastasis[
MATERIALS AND METHODS
Patient selection
Institutional review board approval was obtained for a retrospective review of patients treated from June 2006 to July 2017. Patients underwent clinical evaluations and MRI and CT studies. The three different posterior MIS techniques were employed: posterior tubular decompression and percutaneous instrumented fusion (Subgroup A), tubular partial corpectomy and percutaneous instrumented fusion (Subgroup B), or tubular complete transpedicular or costotransverse corpectomy and percutaneous instrumented fusion (Subgroup C). The decision-making process utilized: Karnofsky performance status (KPS), patient age, neurological deficit, vertebral body involvement, and spinal instability neoplastic score. The 51 patients included, in this study, had thoracic and/or lumbar disease. Patients with previously diagnosed renal cell carcinoma underwent preoperative endovascular tumor embolization. Multiple variables were collected for each patient and are included in [
Fifteen patients (29.4%) were in Subgroup A, 19 (37.2%) in Subgroup B, and 17 (33.3%) in Subgroup C. The average follow-up period was 18.5 ± 24.2 months for all patients. The mean number of levels instrumented was 2, 3, and 4 in Subgroups A-C, respectively [
Surgical approaches
Surgery A: Tubular laminectomy and percutaneous instrumented fusion Patients are placed prone and intraoperative fluoroscopy is used to aid with percutaneous screw placement. When deemed appropriate, polymethylmethacrylate (PMMA) pedicle screw augmentation was employed as previously described[ Surgery B: Partial corpectomy and percutaneous instrumented fusion Screw placement was performed as described above. The unilateral 24 mm diameter tube was used to visualize and remove the lamina and tumor from the dorsal thecal sac, and the transpedicular avenue was utilized to remove bone and tumor anteriorly. If less than 50% of the vertebral body is involved, a partial corpectomy is performed followed by injection of PMMA into the cavity held in place through Steinman pin. Rods are then placed as described above. Surgery C: Complete transpedicular corpectomy and percutaneous instrumented fusion The procedure is performed as described in “Surgery B.” In addition, an expandable port is utilized to perform the majority of the transpedicular corpectomy [
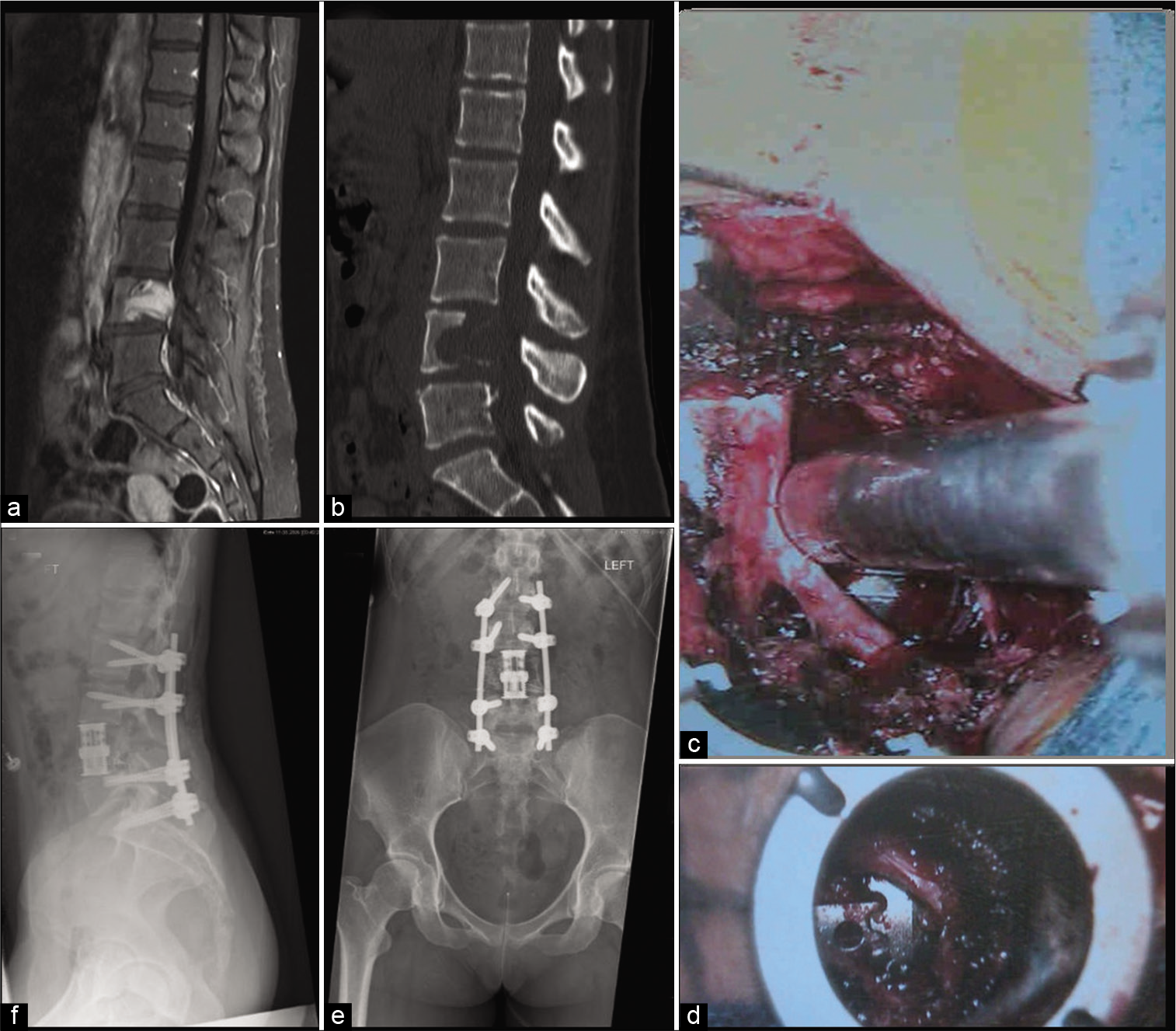
Figure 1:
Case example of metastatic epidural spinal cord compression from a breast cancer primary tumor with low back pain, cauda equina symptoms, and right-sided L4 radiculopathy. (a and b) An MRI T1 sequence with gadolinium is shown with an L4 vertebral body enhancing lesion with canal compression and a predominantly lytic component on sagittal CT. (c) Intraoperatively, an expandable VBR cage is placed after corpectomy and removal of posterior elements through an expandable port. (d) The VBR cage is seen from the contralateral side after further decompression is performed through a 24 mm tube. (e and f) Postoperative X-ray AP and lateral images show L4 VBR cage with L2 to S1 posterior instrumentation.
RESULTS
Preoperative functional status was collected using the Frankel grade and KPS. Patients had a mean preoperative KPS of 70, and overall had a 69% improvement in Frankel grades postoperatively [
Complications
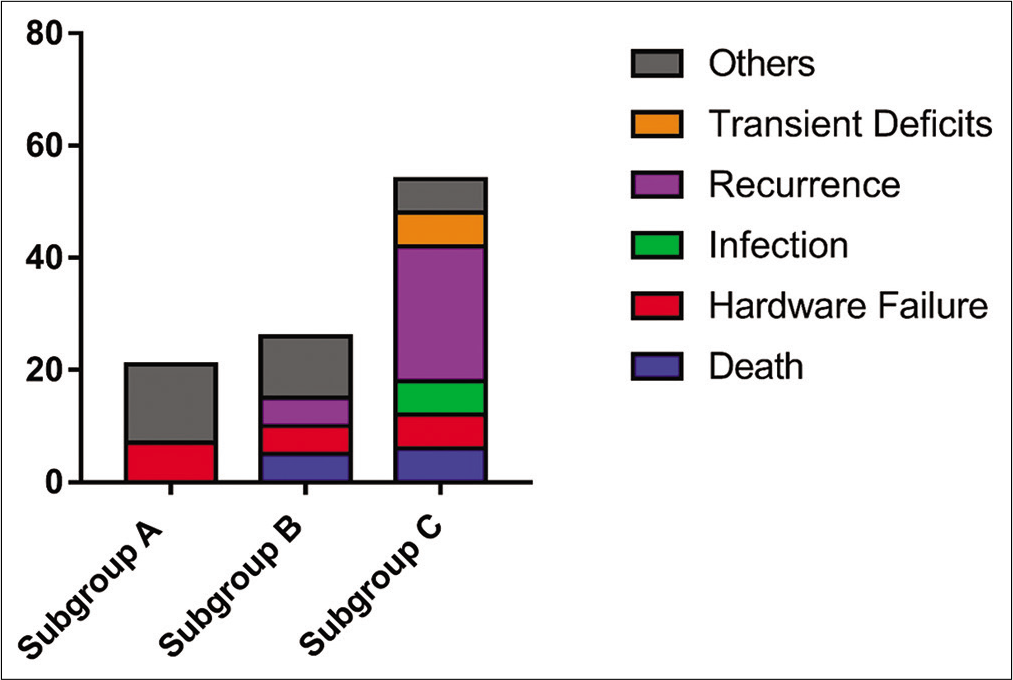
There were 16 postoperative complications 3 (20%) in Subgroup A, 5 (26%) in Subgroup B, and 9 (53%) in Subgroup C [
Figure 3:
Complication rates in Subgroups A, B, and C. There was no statistically significant difference between the groups in complication rates, but there was a trend toward higher complication rates with Subgroup C compared to Subgroups A and B. Other complications include two cases of postoperative ileus, seroma formation requiring re-operation, and one case of pleural effusion requiring chest-tube placement.
DISCUSSION
Spinal epidural metastatic disease develops in up to 36% of patients with malignancy.[
The versatility of MIS approaches has significantly advanced in the past decade and has allowed for the expansion of surgical approaches from less to more aggressive in nature. The least invasive approach is the posterior tubular decompression and percutaneous instrumented fusion (Subgroup A), Next, is the tubular partial corpectomy and percutaneous instrumented fusion (Subgroup B). The most aggressive MIS surgery in this cohort is tubular complete transpedicular or costotransverse corpectomy and percutaneous instrumented fusion (Subgroup C).
Overall functional improvement occurred in all three patients cohorts (e.g., 69%), which compared favorably with published data (20–76.5%),[
CONCLUSION
In our single-institution, MIS cohort, we demonstrate the versatility of various posterior-only MIS approaches for the treatment of MESCC. However, the more aggressive the MIS procedure the greater the complication rates without an improvement in functional outcomes.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Frankel receives royalties from Orthofix and Stryker.
References
1. Bartanusz V, Porchet F. Current strategies in the management of spinal metastatic disease. Swiss Surg. 2003. 9: 55-62
2. Chou D, Lu DC. Mini-open transpedicular corpectomies with expandable cage reconstruction. Technical note. J Neurosurg Spine. 2011. 14: 71-7
3. Fang T, Dong J, Zhou X, McGuire RA, Li X. Comparison of mini-open anterior corpectomy and posterior total en bloc spondylectomy for solitary metastases of the thoracolumbar spine. J Neurosurg Spine. 2012. 17: 271-9
4. Frankel BM, D’Agostino S, Wang C. A biomechanical cadaveric analysis of polymethylmethacrylate-augmented pedicle screw fixation. J Neurosurg Spine. 2007. 7: 47-53
5. Frankel BM, Jones T, Wang C. Segmental polymethylmethacrylate-augmented pedicle screw fixation in patients with bone softening caused by osteoporosis and metastatic tumor involvement: A clinical evaluation. Neurosurgery. 2007. 61: 531-7
6. Georgy BA. Metastatic spinal lesions: State-of-the-art treatment options and future trends. AJNR Am J Neuroradiol. 2008. 29: 1605-11
7. Hikata T, Isogai N, Shiono Y, Funao H, Okada E, Fujita N. A retrospective cohort study comparing the safety and efficacy of minimally invasive versus open surgical techniques in the treatment of spinal metastases. Clin Spine Surg. 2017. 30: E1082-7
8. Klimo P, Schmidt MH. Surgical management of spinal metastases. Oncologist. 2004. 9: 188-96
9. Molina CA, Gokaslan ZL, Sciubba DM. A systematic review of the current role of minimally invasive spine surgery in the management of metastatic spine disease. Int J Surg Oncol. 2011. 2011: 598148
10. Schulte M, Schultheiss M, Hartwig E, Wilke HJ, Wolf S, Sokiranski R. Vertebral body replacement with a bioglass-polyurethane composite in spine metastases-clinical, radiological and biomechanical results. Eur Spine J. 2000. 9: 437-44
11. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005. 30: 2186-91
12. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001. 26: 298-306
13. Wong DA, Fornasier VL, MacNab I. Spinal metastases: The obvious, the occult, and the impostors. Spine (Phila Pa 1976). 1990. 15: 1-4