- Department of Neurosurgery, Kyoto Prefectural University of Medicine Graduate School of Medical Science, Kyoto, Japan
- Department of Neurosurgery, National Hospital Organization Maizuru Medical Center, Maizuru, Japan
- Department of Biostatistics, Kyoto Prefectural University of Medicine Graduate School of Medical Science, Kyoto, Japan.
Correspondence Address:
Takanari Okamoto, Department of Neurosurgery, Kyoto Prefectural University of Medicine Graduate School of Medical Science, Kyoto, Japan.
DOI:10.25259/SNI_820_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takanari Okamoto1, Yasuo Inoue2, Yuta Oi1, Ichita Taniyama1, Takashi Houri2, Satoshi Teramukai3, Naoya Hashimoto1. Strategy of carotid artery stenting as first-line treatment and carotid endarterectomy for carotid artery stenosis: A single-center experience. 04-Nov-2022;13:513
How to cite this URL: Takanari Okamoto1, Yasuo Inoue2, Yuta Oi1, Ichita Taniyama1, Takashi Houri2, Satoshi Teramukai3, Naoya Hashimoto1. Strategy of carotid artery stenting as first-line treatment and carotid endarterectomy for carotid artery stenosis: A single-center experience. 04-Nov-2022;13:513. Available from: https://surgicalneurologyint.com/surgicalint-articles/11972/
Abstract
Background: The main surgical options for stenosis of the carotid artery are carotid endarterectomy (CEA) and carotid artery stenting (CAS). The number of CAS procedures performed in Japan greatly exceeds that of CEA procedures. In this study, we used data from a single center to examine CAS and CEA for carotid artery stenosis.
Methods: The subjects were patients with carotid artery stenosis who underwent CAS or CEA between January 2012 and May 2020. CAS was the first-choice treatment. CEA was used in cases with vulnerable plaques, a relatively low risk of general anesthesia, and no anatomical features disadvantageous for endarterectomy.
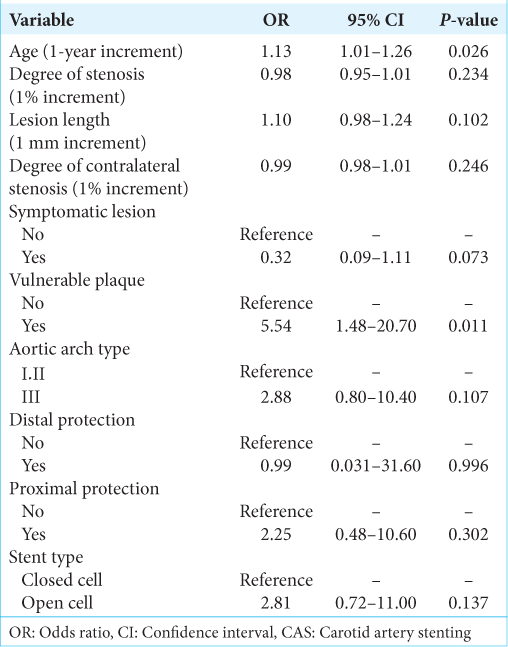
Results: A total of 140 cases (102 CAS and 38 CEA) were examined. There were more elderly patients in the CAS group. The CEA group had a higher rate of vulnerable plaques and only one case with an unfavorable anatomy for CEA. Major adverse events (stroke) occurred in two CAS cases. In multivariate logistic analysis, postoperative ischemic lesions were independently associated with age (odds ratio [OR] = 1.13, 95% confidence interval [CI]: 1.01–1.26, P = 0.026) and vulnerable plaque (OR = 5.54, 95% CI: 1.48–20.70, P = 0.011) in the CAS group, but not in the CEA group.
Conclusion: The results reflect the treatment algorithm at our hospital, indicating that triage is accurate. Thus, it is beneficial to assign cases based primarily on plaque vulnerability and anatomical risk for CEA, and to not hesitate to perform CEA simply because of old age. CAS as first-line treatment and CEA are effective and safe, which reflect the treatment situation in Japan.
Keywords: Carotid artery stenting, Carotid endarterectomy, Major adverse event, Postoperative ischemic lesion, Treatment strategy
INTRODUCTION
Carotid endarterectomy (CEA) and carotid artery stenting (CAS) are well-established treatments for symptomatic or asymptomatic stenosis of the carotid artery.[
There are opposing opinions on CEA and CAS, but, in recent years, this discussion has focused on selection of appropriate treatment based on the characteristics of both procedures. The unique feature of surgical treatment for carotid artery stenosis in Japan is that both CAS and CEA can be performed by the same team or the same neurosurgeon. Therefore, treatment options tend to be selected more evenly than in other countries and the outcomes of both treatments are likely to be good. In this study, we analyzed data from a single center to examine the strategy of CAS as first-line treatment and CEA for carotid artery stenosis.
MATERIALS AND METHODS
Study design and patients
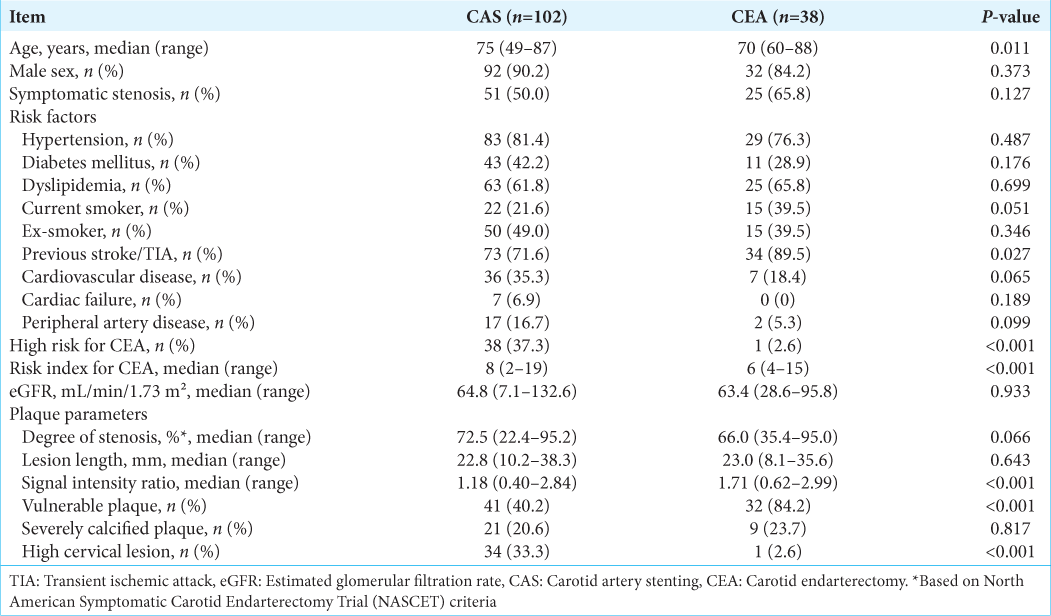
Patients with carotid artery stenosis who underwent CAS or CEA at our hospital between January 2012 and May 2020 were reviewed retrospectively in a single-center study. Surgical treatment was performed for patients with (1) angiographically symptomatic stenosis of at least 50% of the luminal diameter, (2) asymptomatic stenosis of at least 70%, and (3) symptomatic stenosis with intraluminal ulceration. Patients who were unable to complete the surgical treatment and those with occlusion or dissection were excluded from the study. Demographic information (age, sex, and race), symptomatic stenosis, stroke risk factors (smoking status, hypertension, diabetes mellitus, hyperlipidemia, history of stroke, and history of heart disease), renal function (estimated glomerular filtration rate), plaque parameters (degree of stenosis and lesion length), pre- and postoperative modified Rankin Score (mRS), and postoperative complications were collected for each patient. Carotid stenosis was deemed symptomatic if neurologic symptoms attributable to the ipsilateral hemisphere were present within a year before presentation.
Preoperative evaluation and patient selection
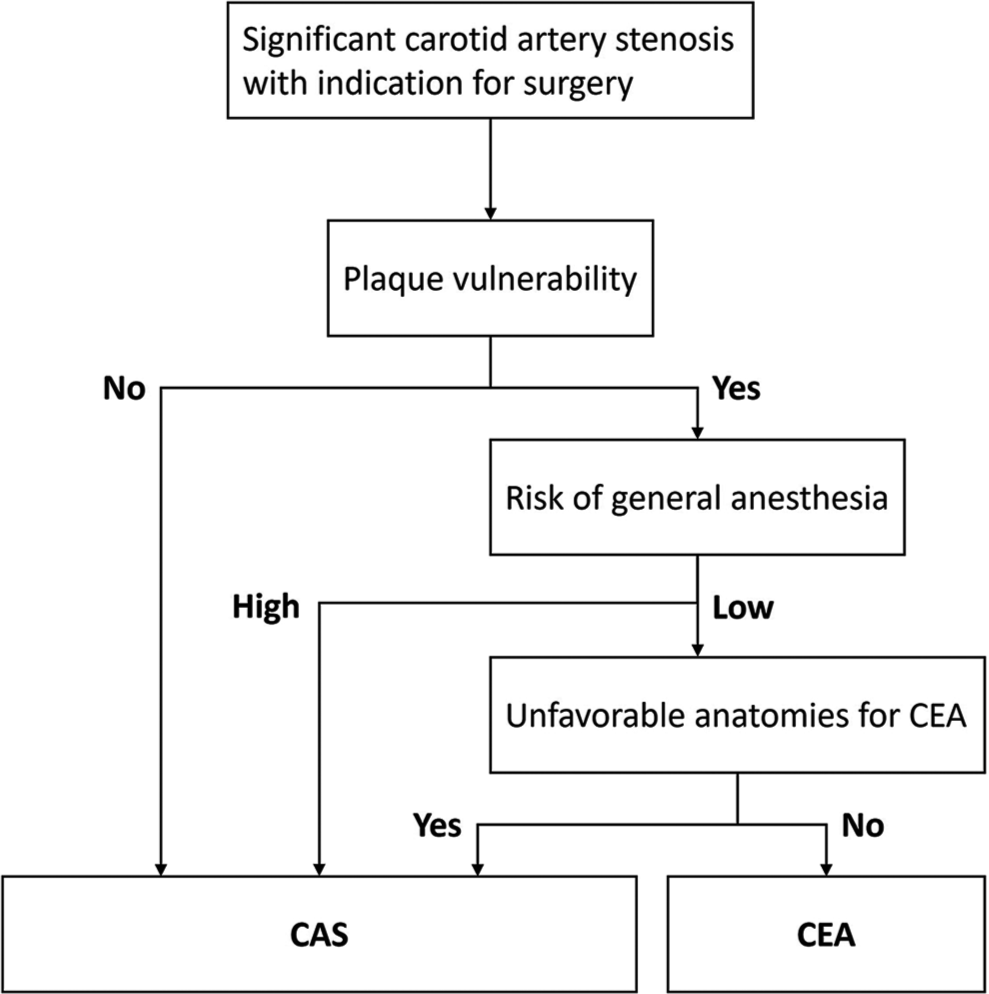
For patients with carotid artery stenosis diagnosed by ultrasonography (US), either CTA or diagnostic angiography was performed before intervention to identify anatomical factors that could affect the treatment decision. Preoperative US, MRI, and CTA were used to evaluate internal carotid artery (ICA) plaques. In addition to carotid imaging, blood pressure monitoring, heart rhythm monitoring, electrocardiogram, and echocardiography were performed. If there were any abnormalities in cardiac function, the patient was examined by a cardiologist and prioritized for cardiac treatment. At our hospital, CAS is the first choice for patients with carotid artery stenosis. CEA was chosen in cases with vulnerable plaques; a relatively low risk for general anesthesia, such as cardiopulmonary insufficiency; and no anatomical features disadvantageous for endarterectomy, such as high or low cervical lesions, previous radiotherapy, and contralateral internal carotid stenosis [
Definitions of preoperative variables
Plaque was judged to be vulnerable in a case with a signal intensity ratio (SIR) of carotid plaque against sternocleidomastoid muscle on black-blood fat-suppressed (FS) T1-weighted magnetic resonance imaging (T1-MRI) >1.5 or a high-intensity signal on time-of-flight-MR angiography. In addition, if preoperative US of the ICA plaque showed a mobile component that was not synchronized with the heartbeat, the plaque was also judged to be vulnerable.[
Surgical procedures
CAS was performed under local anesthesia. Patients who underwent CAS received perioperative dual antiplatelet therapy (usually aspirin and clopidogrel) and intraoperative systemic heparinization. An embolic protection device was utilized in all cases, and dual protection (proximal balloon protection of the common carotid artery and the external carotid artery, and distal protection) was used whenever possible. Distal protection was mainly performed with filter devices. An open- or closed-cell stent was selected depending on plaque vulnerability and flexion of the lesion. For example, we primarily chose a closed-cell stent for linear lesions with vulnerable plaque. Systemic heparinization was continued for 5 min after the final angioplasty and not reversed.
CEA was performed under general anesthesia. Preoperative single antiplatelet therapy (usually aspirin) was continued, and systemic heparin was administered before blood flow was blocked. Patients underwent monitoring with continuous electroencephalography and near-infrared spectroscopy. Three-way internal shunts were placed in almost all cases. No patch angioplasty was performed.
Postoperative evaluation
Complications associated with CEA or CAS and those common to both, such as hyperperfusion, were assessed. Postoperative stroke or asymptomatic hyperintensities on diffusion-weighted imaging (DWI) were examined within 48 h after surgery. Major adverse events were defined as major stroke, myocardial infarction, and death after surgical treatment. Stroke was defined as a rapidly developing clinical syndrome of focal disturbance of cerebral function that lasted more than 24 h. Minor and major strokes were defined as new neurological deficits that completely resolved within 30 days or lasted for more than 30 days of follow-up, respectively. The mRS score for disability was used to evaluate the preoperative and 1-year postoperative neurological conditions of the patients.
Statistical analysis
For patient characteristics, continuous and ordinal variables are summarized as median and range and compared by Mann–Whitney U-test, and categorical variables are summarized as frequencies (percentages) and compared by Fisher’s exact test. Relationships between the occurrence of postoperative ischemic lesions, including symptomatic and asymptomatic lesions positive on DWI, and patient characteristics were examined by multivariate logistic regression analysis according to the surgical procedures (CAS and CEA). P < 0.05 was considered to be statistically significant. All statistical analyses were performed with R version 4.0.0 (The R Foundation for Statistical Computing).
RESULTS
Patient background
After a retrospective review of cases, three patients were excluded from the study due to pseudo-occlusion with the guidewire unable to pass through, complete occlusion with the guidewire failing to pass, and performance of CAS for an occlusion due to carotid artery dissection, respectively. Of the 140 cases enrolled in the study, 102 underwent CAS and 38 underwent CEA. All of the patients were Japanese. The characteristics of the 140 cases are shown in
Postoperative clinical outcome
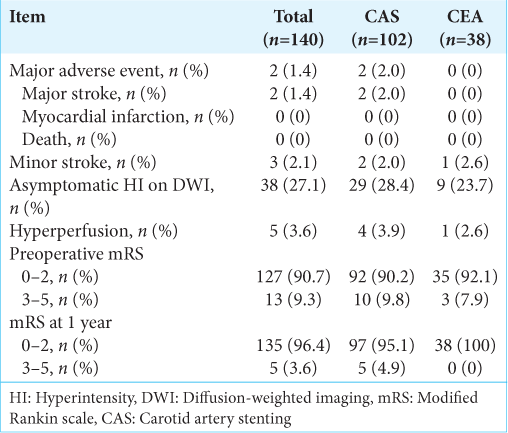
Postoperative clinical outcomes after CAS or CEA are shown in
Postoperative ischemic lesions after CAS or CEA
Symptomatic or asymptomatic lesions positive on DWI within 48 h after surgery were observed in 33 cases (32.4%) in the CAS group and 10 cases (26.3%) in the CEA group. The adjusted odds ratio (OR) with 95% confidence intervals (CIs) for positive DWI after CAS is shown in
Illustrative case: A patient with major stroke
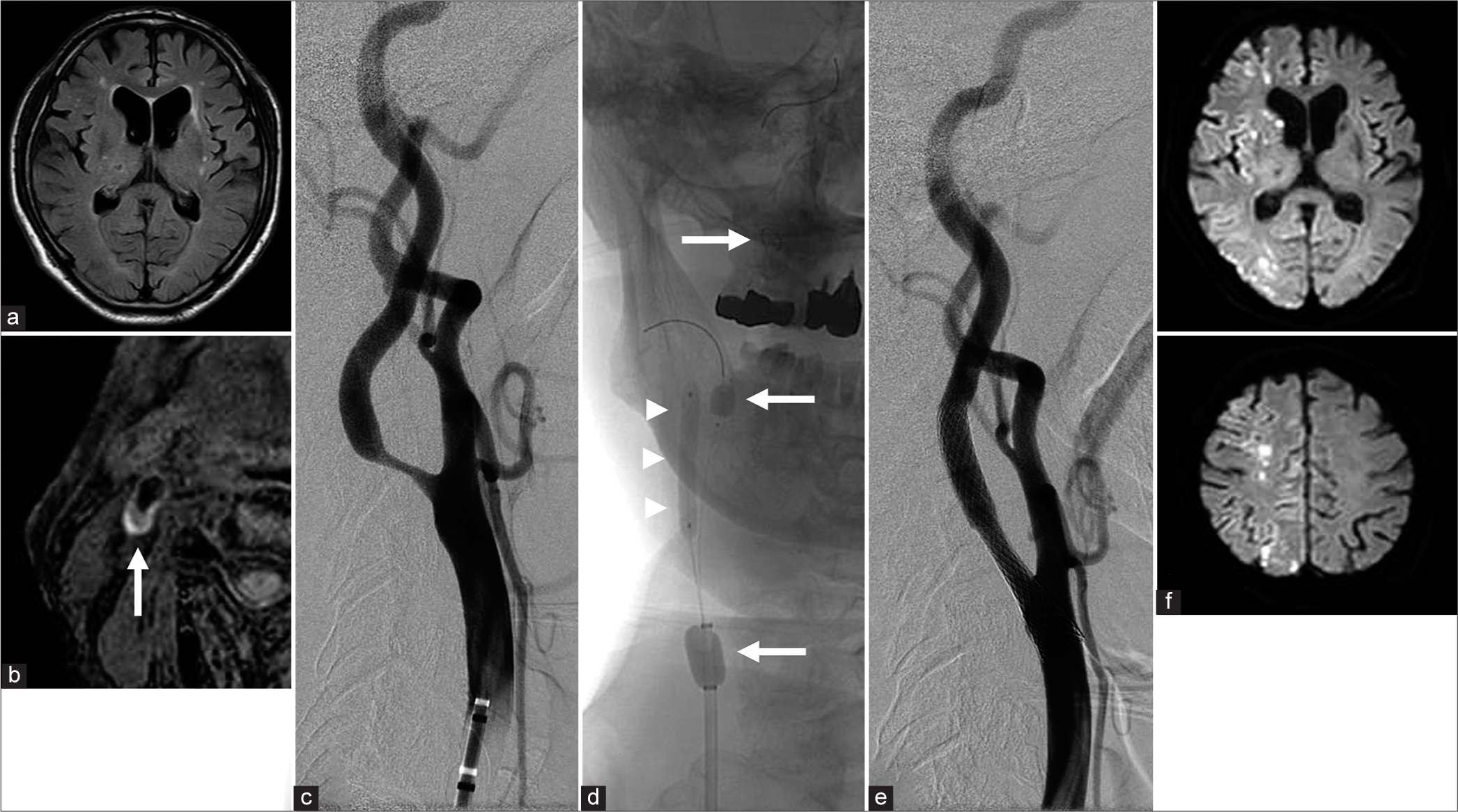
The patient was a 78-year-old man with asymptomatic right ICA stenosis who was indicated for surgical treatment because the stenosis was >70%, and further, progression was observed. Preoperative fluid-attenuated inversion-recovery MRI showed chronic ischemic white matter changes and no acute changes [
Figure 2:
(a) Preoperative fluid-attenuated inversion-recovery MRI showing chronic ischemic white matter changes and no acute changes. (b) Black-blood fat-suppressed T1-weighted MRI showed high-intensity signals for internal carotid artery (ICA) plaque (white arrow). (c) Preoperative lateral subtraction angiogram showing severe stenosis in the right ICA. (d) Frontal unsubtracted angiographic view during carotid artery stenting (CAS) using dual protection (proximal balloon and distal filter) (white arrows). Under dual protection, predilation was performed with a 4×30 mm angioplasty catheter (white arrowheads). (e) Postoperative lateral view of a digital subtraction angiogram shows a dilated stenotic lesion after CAS. Postoperative diffusion-weighted MRI showed multiple infarction in the right hemisphere.
DISCUSSION
The characteristics of the patients show that the results of this study reflect the treatment algorithm used at our facility, indicating that triage is accurate. In the CAS group, adequate selection of protection devices and surgical procedure can produce satisfactory results, and combined with CEA, the rate of major adverse events was very low. However, vulnerable plaques were associated with increased risk of postoperative stroke after CAS, but not after CEA. These results are consistent with the high incidence of cerebral ischemic complications in CAS for vulnerable plaques with high signal intensity on MRI.[
All patients with major stroke in this study had preoperative findings of possible vulnerable plaque, but some also had factors that discouraged CEA under general anesthesia, such as advanced age and a high cervical lesion, so CAS was chosen. CAS for vulnerable plaques requires stringent embolic protection, and combined proximal and distal protection might be more useful and safer than single protection alone.[
Multivariate logistic analysis revealed that proximal protection was weakly correlated with a risk of postoperative ischemic lesions, but strongly correlated with the aortic arch type. Instrumentation of an atherosclerotic aortic arch may release plaque debris to the brain during carotid catheterization. A balloon-guided catheter has a larger diameter and higher rigidity than a nonballoon-guided catheter, and proximal balloon protection involves more complex surgical procedures. Increased shear stress in the artery walls during carotid catheterization and the longer procedure time may affect the results.[
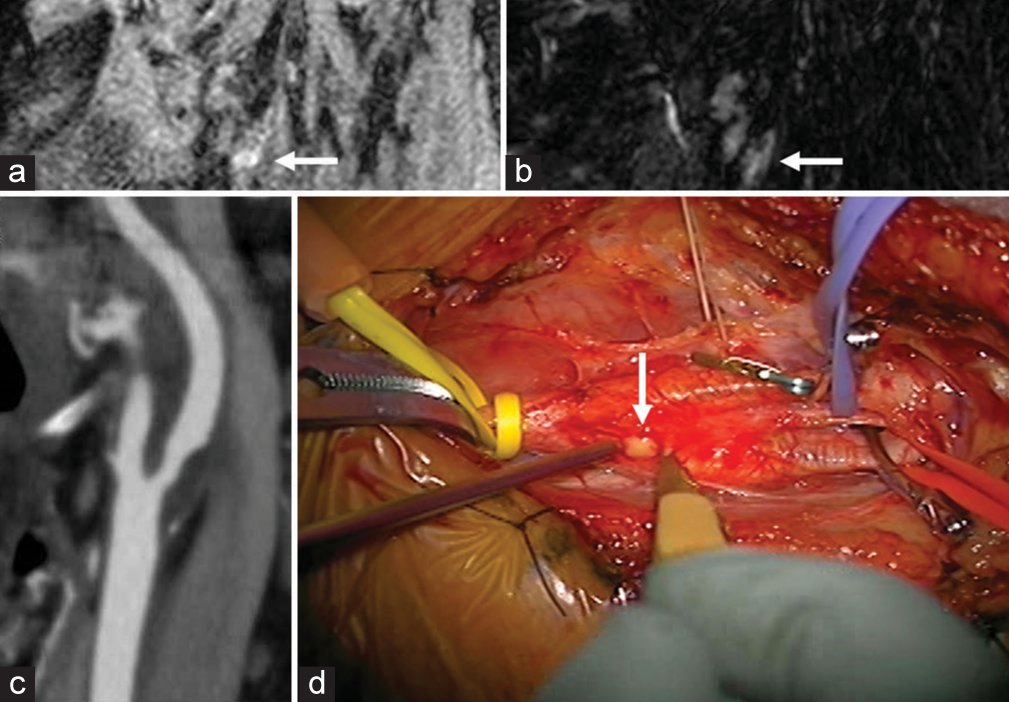
In some CEA cases, viscous fluid flowed out from the carotid plaque after cutting the adventitia of the common carotid artery [
Figure 3:
(a and b) Long-axis black-blood fat-suppressed T1- and T2-weighted MRI showing high-intensity signals for internal carotid artery (ICA) plaque (white arrows). (c) CT angiography indicated approximately 50% stenosis using the criteria defined in the North American Symptomatic Carotid Endarterectomy Trial (NASCET). (d) Viscous fluid flowed from the carotid plaque after cutting of the adventitia of the common carotid artery (white arrow).
The Carotid Revascularization Endarterectomy versus Stent Trial demonstrated superiority of CEA in elderly patients. Outcomes were slightly better after CAS for patients aged <70 years and better after CEA for patients >70 years.[
In recent years, the use of CAS has increased markedly due to advantages of convenience, less invasiveness, and advances in devices. In 2019, 8471 CAS and 4053 CEA procedures were performed in Japan, and the proportion of CAS is increasing yearly.[
Two of our patients (1.4%) had major stroke and 3 (2.1%) had minor stroke, indicating that our results are not inferior to those of JR-NET3 or ACST-2. Our algorithm reflects the treatment situation in Japan, where the proportion of CAS procedures is increasing, and we obtained relatively good outcomes. There are conflicting views of CAS and CEA, but the key issue for good outcomes seems to be use of an appropriate treatment algorithm. A strategy using CAS as first-line treatment and CEA based on individual factors, with performance of the two procedures by the same team, appears to be reasonable.
This study has several limitations. First, it was a single-center retrospective study with no control group, and the statistical power was low due to the small number of patients. Second, there was no uniform algorithm used for medical treatment and the algorithm for surgical treatment was not sufficiently rigorous, especially in determining the risks of general anesthesia. In addition, the follow-up period was short and evaluation of long-term outcomes for events such as restenosis and recurrent stroke is required.
CONCLUSION
CAS as first-line treatment and CEA are effective and safe, and this strategy reflects the treatment situation in Japan. It is beneficial to assign cases to CEA based primarily on plaque vulnerability and anatomical risk, but the indications and procedures can be modified and it seems to be important to not hesitate to perform CEA just because of old age.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011. 124: e54-130
2. Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010. 363: 11-23
3. Castro-Afonso LH, Abud LG, Rolo JG, Santos AC, Oliveira LD, Barreira CM. Flow reversal versus filter protection: A pilot carotid artery stenting randomized trial. Circ Cardiovasc Interv. 2013. 6: 552-9
4. Flach HZ, Ouhlous M, Hendriks JM, Van Sambeek MR, Veenland JF, Koudstaal PJ. Cerebral ischemia after carotid intervention. J Endovasc Ther. 2004. 11: 251-7
5. Gates L, Botta R, Schlosser F, Goodney P, Fokkema M, Schermerhorn M. Characteristics that define high risk in carotid endarterectomy from the Vascular Study Group of New England. J Vasc Surg. 2015. 62: 929-36
6. Halliday A, Bulbulia R, Bonati LH, Chester J, CradduckBamford A, Peto R. Second asymptomatic carotid surgery trial (ACST-2): A randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet. 2021. 398: 1065-73
7. Howard G, Roubin GS, Jansen O, Hendrikse J, Halliday A, Fraedrich G. Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: A meta-analysis of pooled patient data from four randomised trials. Lancet. 2016. 387: 1305-11
8. Howie BA, Witek AM, Hussain MS, Bain MD, Toth G. Carotid endarterectomy and carotid artery stenting in a predominantly symptomatic real-world patient population. World Neurosurg. 2019. 127: e722-6
9. Iihara K, Saito N, Suzuki M, Date I, Fujii Y, Houkin K. The Japan neurosurgical database: Statistics update 2018 and 2019. Neurol Med Chir (Tokyo). 2021. 61: 675-710
10. Kajihara Y, Sakamoto S, Kiura Y, Mukada K, Chaki T, Kajihara S. Comparison of dual protection and distal filter protection as a distal embolic protection method during carotid artery stenting: A single-center carotid artery stenting experience. Neurosurg Rev. 2015. 38: 671-6
11. Kubota H, Sanada Y, Yoshioka H, Tasaki T, Shiroma J, Miyauchi M. C1 transverse process-hyoid bone line for preoperative evaluation of the accessible internal carotid artery on carotid endarterectomy: Technical note. Acta Neurochir (Wien). 2015. 157: 43-8
12. Macdonald S, Lee R, Williams R, Stansby G. Towards safer carotid artery stenting: A scoring system for anatomic suitability. Stroke. 2009. 40: 1698-703
13. Moody AR, Allder S, Lennox G, Gladman J, Fentem P. Direct magnetic resonance imaging of carotid artery thrombus in acute stroke. Lancet. 1999. 353: 122-3
14. Mutzenbach JS, Griessenauer CJ, Broussalis E, Pikija S, Moscote-Salazar LR, Millesi K. Follow-up after carotid stenting with the CASPER stent system: A duplex ultrasound evaluation. J Vasc Surg. 2020. 72: 2054-60.e2
15. Park JH, Lee JH. Carotid artery stenting. Korean Circ J. 2018. 48: 97-113
16. . Report of Japan Neurosurgery Registry (2015-2017). Neurol Med Chir (Tokyo). 2019. 59: 13-81
17. Sakamoto S, Kiura Y, Okazaki T, Shinagawa K, Ishii D, Ichinose N. Carotid artery stenting for vulnerable plaques on MR angiography and ultrasonography: Utility of dual protection and blood aspiration method. J Neurointerv Surg. 2016. 8: 1011-5
18. Tokuda R, Yoshimura S, Uchida K, Yamada K, Satow T, Iihara K. Real-world experience of carotid artery stenting in Japan: Analysis of 8458 cases from the JR-NET3 nationwide retrospective multi-center registries. Neurol Med Chir (Tokyo). 2019. 59: 117-25
19. Watanabe Y, Nagayama M, Suga T, Yoshida K, Yamagata S, Okumura A. Characterization of atherosclerotic plaque of carotid arteries with histopathological correlation: Vascular wall MR imaging vs. color Doppler ultrasonography (US). J Magn Reson Imaging. 2008. 28: 478-85
20. Yoshida K, Endo H, Sadamasa N, Narumi O, Chin M, Inoue K. Evaluation of carotid artery atherosclerotic plaque distribution by using long-axis high-resolution black-blood magnetic resonance imaging. J Neurosurg. 2008. 109: 1042-8
21. Yoshimura S, Yamada K, Kawasaki M, Asano T, Kanematsu M, Takamatsu M. High-intensity signal on time-of-flight magnetic resonance angiography indicates carotid plaques at high risk for cerebral embolism during stenting. Stroke. 2011. 42: 3132-7