- Department of Neurosurgery, Ramón y Cajal University Hospital, Madrid, Spain.
- Department of Pathology, Ramón y Cajal University Hospital, Madrid, Spain.
- Department of Radiology, Ramón y Cajal University Hospital, Madrid, Spain.
Correspondence Address:
María López Gutiérrez, Department of Neurosurgery, Ramón y Cajal University Hospital, Madrid, Spain.
DOI:10.25259/SNI_336_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: María López Gutiérrez1, Rodrigo Carrasco-Moro1, Ignacio Ruz-Caracuel2, Juan S. Martínez San Millán3. Stroke secondary to leptomeningeal carcinomatosis with radiologic signs of arterial invasion. 08-Jul-2022;13:290
How to cite this URL: María López Gutiérrez1, Rodrigo Carrasco-Moro1, Ignacio Ruz-Caracuel2, Juan S. Martínez San Millán3. Stroke secondary to leptomeningeal carcinomatosis with radiologic signs of arterial invasion. 08-Jul-2022;13:290. Available from: https://surgicalneurologyint.com/surgicalint-articles/11709/
Abstract
Background: Compared to the general population, cancer patients are more likely to suffer from cerebral ischemia, either caused by the tumor itself or by the treatments applied.
Case Description: We hereby present the clinical case of a patient treated for lung adenocarcinoma, who, years later, developed a case of the right frontal-temporal-insular ischemia secondary to leptomeningeal spread of the primary neoplasm, with an invasion of the walls of the right-middle cerebral artery and its branches.
Conclusion: This should be considered an extremely rare form of recurrence of a primary solid tumor with clinical and radiological features that can mimic those of vascular inflammatory entities.
Keywords: Arterial wall metastasis, Brain metastasis, Leptomeningeal carcinomatosis, Stroke
INTRODUCTION
Approximately 15% of cancer patients suffer from cerebrovascular events, either ischemic or hemorrhagic in nature, while 4.4% of stroke patients also show signs of active neoplastic disease.[
CLINICAL PRESENTATION
Presentation and examination
A 76-year-old woman had been treated 6 years ago for a Stage IA lung adenocarcinoma (T1aN0M0; TTF1+ and thyroglobulin-immunostaining; nonrearranged ALK gene locus; nonmutated K-RAS, BRAF, and EGFR) by the left upper pulmonary lobectomy and mediastinal lymphadenectomy. She remained free of disease according to the records of their last cancer follow-up appointment. The patient was transferred to the emergency department with a clinical picture of a headache, self-limited and recurrent episodes of dizziness, unsteady gait and diaphoresis, and occasional loss of consciousness over the past few months. She presented unsteady gait with lateralization to the left, without additional signs of focal involvement in the neurological examination. Since the patient also had a history of arterial hypertension, dyslipidemia, and ischemic heart disease, she had recently been evaluated as an outpatient by her cardiologist, who ruled out any structural or functional pathology that would justify her current clinical picture.
Complementary studies
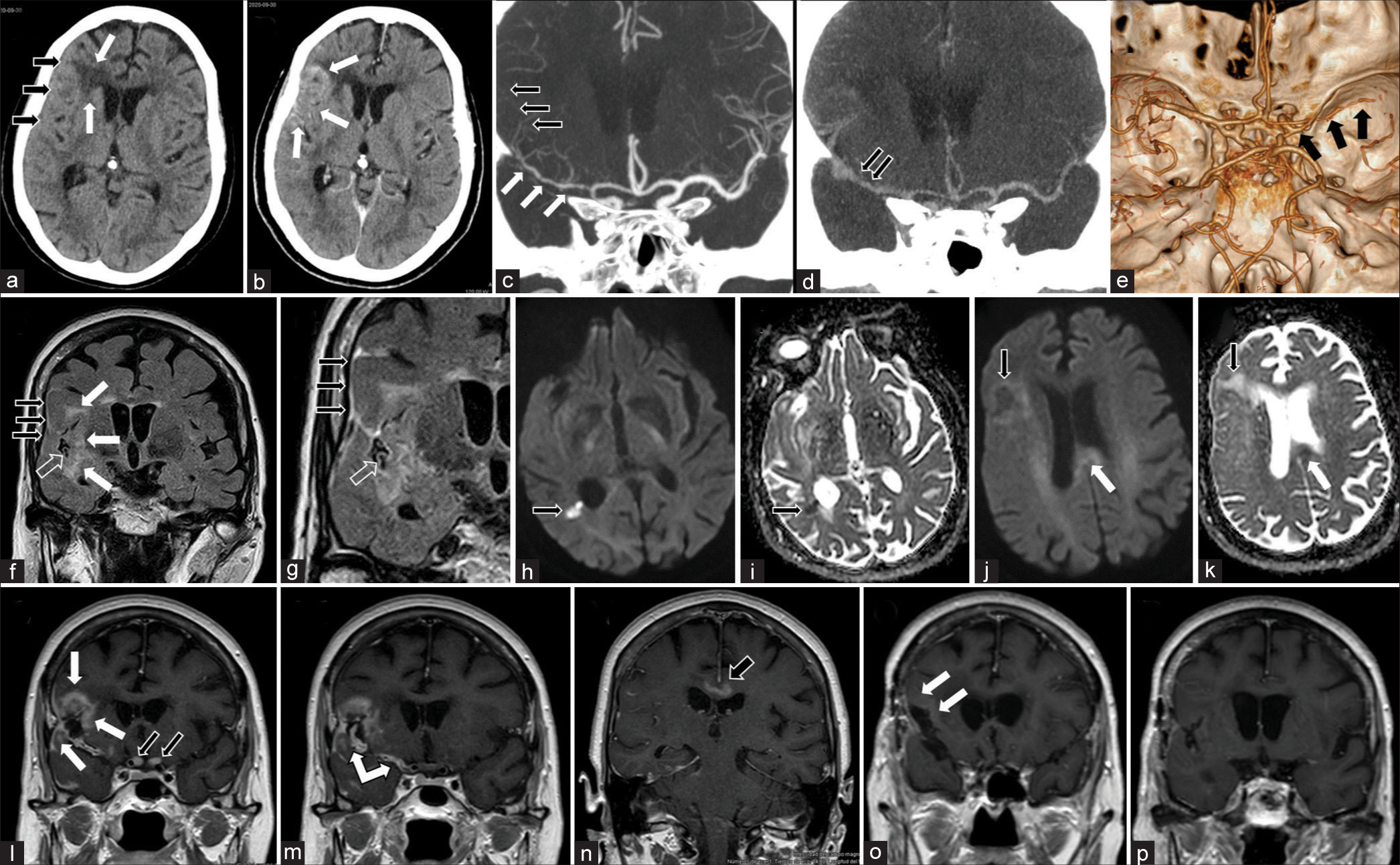
The analysis of peripheral blood returned nominal measurements. Imaging studies revealed signs of ischemia in different stages affecting the right insula together with the frontal and temporal opercula, frontal lobe white matter edema, and LMC with an invasion of the right middle cerebral arterial wall and its branches [
Figure 1:
Diagnostic imaging gallery. (a and b) Emergent brain CT. (a) This baseline study shows cortical thickening with sulcus collapse in the right frontotemporal region (black arrows), and an area of low density involving the adjacent frontal white matter (white arrows). (b) Focal gyriform enhancement can be observed (white arrows) after the administration of intravenous contrast. (c-e) Coronal (c and d) and 3D (e) reconstructions of a cerebral CTA study obtained immediately (c and e) and 4 min after contrast administration (d). The former reveals a striking multisegmental luminal stenosis, with narrowing of the right middle cerebral arterial tree (white arrows in c and black arrows in e) and distal vascular occlusion (black arrows in c); the late-phase study (d) shows prominent contrast deposits (black arrows) in said arterial structures. (f-n) Preoperative brain MRI study.(f) Coronal slices corresponding to the FLAIR sequence that demonstrates an abnormally increased signal coating the opercular brain surface (black arrows) and the arterial vessels running through an unoccupied Sylvian cistern (transparent arrow); also notice the signal alteration involving the right insular, frontal, and temporal parenchyma (white arrows); (g) Coronal slice corresponfing to FLAIR sequence obtained after administration of paramagnetic contrast. It demonstrates abnormally increased sign at the opercular brain surface (black arrows), and the arterial vessels through the Sylvian cistern (transparent arrow). (h-k) Diffusion-weighted MRI slices (h and j) displaying increased signal in the frontal lobe (black arrow in j), temporal, periventricular area (black arrow in h), and corpus callosum (white arrow in j); the two latters show signal restriction in the corresponding ADC maps (i and k), restriction periventricular area (black arrow in i) k) note the corresponding alterations in the frontal lobe (black arrow) and periventricular area (white arrow). (l-n) T1 sequence after the administration of gadolinium coronal sections is shown in a sequential, anteroposterior order – reveals irregular opercular and insular gyral enhancement (white arrows in l), diffuse and irregular thickening of the walls of the right middle cerebral artery from its origin (double-headed white arrow in m), a homogeneous uptake of both optic nerves (black arrows in l), and irregular enhancement in the corpus callosum (black arrow in n). (o-p) Control MRI study obtained after oncologic treatment; these coronal slices from the T1W sequence after administration of paramagnetic contrast, demonstrate no enhancement of previously affected structures with faint, apparently residual, and right frontal leptomeningeal uptake (white arrows in o).
Histological diagnosis
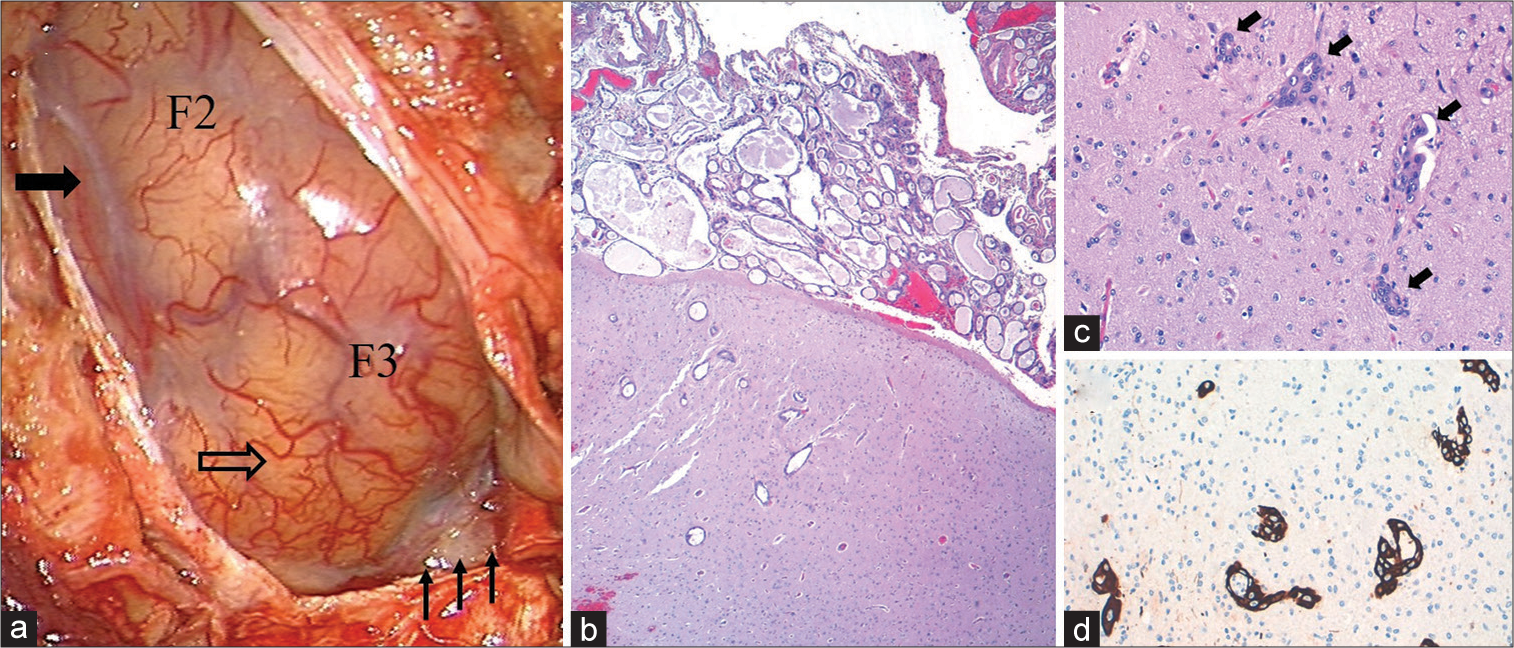
Finally, an open brain biopsy was performed [
Figure 2:
Macro- and microscopic pathological findings. (a) Surgical photograph obtained after performing a right frontal craniotomy, with partial exposure of the second (F2) and third (F3) convolutions. The opercular area presents an apparently normal superficial vascular pattern (transparent arrow), while the dorsal area of the exposed surgical field presents a hypovascular appearance (solid arrow); note the opacity of the Sylvian cistern and some neighboring sulci that display a grayish-yellowish coloration (small arrows in the lower right margin). (b) Low magnification view of the biopsy specimen showing epithelial cell proliferation with glandular differentiation, together with diffuse leptomeningeal infiltration and multiple cortical neoplastic foci (HE, ×4). (c) At higher magnification, it is observed that the latters (arrows) are arranged inside spaces delimited by endothelium (HE, ×20). (d) Intense staining for cytokeratin 7, denoting the epithelial nature of the tumor cells (CK7, ×20).
Evolution
According to the findings in diagnostic tests carried out, a Stage IVA neoplastic disease was established. The patient received two cycles of treatment with capmatinib in monotherapy (400 mg/12 h), indicated after the identification through next-generation sequencing studies of neoplastic variants (MET splice site 2888-15_2891del19) sensitive to the aforementioned drug in the biopsy sample. The initial response was favorable, manifesting neuroradiological improvement in the MRI control study obtained 4 months after the surgical procedure [
DISCUSSION
LMC is a rare and severe complication which can manifest over the course of an oncological disease’s lifespan.[
LMC usually develops as a consequence of hematogenous dissemination of the primary tumor, but neoplastic cells can also spread to contiguous structures after infiltration of perivascular spaces, and/-or perineural spaces, the ependyma, and/-or the choroid plexuses.[
From the physiopathological point of view, as LMC is characterized by neoplastic infiltration of the pia-arachnoid membranes and the perivascular and perineural spaces, the typical clinical presentation usually includes cranial nerve involvement; nevertheless, dissemination through the CSF may lead to a diffuse or multifocal disease characterized by symptoms of meningeal irritation and/or intracranial hypertension due to hydrocephalus.[
Despite the presence of characteristic findings in neuroimaging studies,[
Although the prognosis for LMC is dire,[
CONCLUSION
Infiltration of the wall of cerebral arteries by tumor cells is an extremely rare phenomenon that can lead to atypical clinical presentations. These may include ischemic events which, in the absence of other evidences of the underlying oncological disease or highly suggestive radiological signs, can either delay the diagnosis or lead to misdiagnosis, with a direct impact on the final clinical prognosis of the patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to express their gratitude to the staff working at the hospital medical library. They are especially grateful to Prof Mr. Ian Fitzpatrick who kindly revised the English version of this manuscript.
References
1. Adams HP. Cancer and cerebrovascular disease. Curr Neurol Neurosci Rep. 2019. 19: 73
2. Berghoff AS, Preusser M. Role of the blood-brain barrier in metastatic disease of the central nervous system. Handb Clin Neurol. 2018. 149: 57-66
3. Bhambhvani HP, Rodrigues AJ, Umeh-Garcia MC, Hayden Gephart M. Leptomeningeal carcinomatosis: Molecular landscape, current management, and emerging therapies. Neurosurg Clin N Am. 2020. 31: 613-25
4. Cowen RL, Siqueira EB, George E. Angiographic demonstration of a glioma involving the wall of the anterior cerebral artery. Report of a case. Radiology. 1970. 97: 577-8
5. Grisold W, Oberndorfer S, Struhal W. Stroke and cancer: A review. Acta Neurol Scand. 2009. 119: 1-16
6. Gutmann DH, Cantor CR, Piacente GJ, McCluskey LF. Cerebral vasculopathy and infarction in a woman with carcinomatous meningitis. J Neurooncol. 1990. 9: 183-5
7. Herman C, Kupsky WJ, Rogers L, Duman R, Moore P. Leptomeningeal dissemination of malignant glioma simulating cerebral vasculitis. Case report with angiographic and pathological studies. Stroke. 1995. 26: 2366-70
8. Kastenbauer S, Wiesmann M, Pfister HW. Cerebral vasculopathy and multiple infarctions in a woman with carcinomatous meningitis while on treatment with intrathecal methotrexate. J Neurooncol. 2000. 48: 41-5
9. Kawaguchi T, Tobai S, Nakamura K. Extravascular migration of tumor cells in the brain: An electron microscopic study. Invasion Metastasis. 1982. 2: 40-50
10. Klein P, Haley EC, Wooten GF, VandenBerg SR. Focal cerebral infarctions associated with perivascular tumor infiltrates in carcinomatous leptomeningeal metastases. Arch Neurol. 1989. 46: 1149-52
11. Le Rhun E, Taillibert S, Chamberlain M. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg Neurol Int. 2013. 4: 265-88
12. Leeds NE, Rosenblatt R, Zimmerman HM. Focal angiographic changes of cerebral lymphoma with pathologic correlation. A report of two cases. Radiology. 1971. 99: 595-9
13. Moore MT, Eisinger G. Extra primary seeding of glioblastoma multiforme in the subarachnoid space and ependyma. Neurology. 1963. 13: 855-65
14. Olson ME, Chernik NL, Posner JB. Infiltration of the leptomeninges by systemic cancer. A clinical and pathologic study. Arch Neurol. 1974. 30: 122-37
15. Osborn AG, Osborn AG.editors. Leptomeningeal metastases. Osborn’s Brain, Imaging, Pathology, and Anatomy. Canada: AMIRSYS; 2013. p. 758-60
16. Patel AJ, Lang FF, Suki D, Wildrick DM, Sawaya R.editors. Metastatic brain tumors. Youmans and Winn Neurological Surgery. Amsterdam, Netherlands: Elsevier Inc; 1989. 4: 1091-106.e5
17. Rogers LR. Cerebrovascular complications in patients with cancer. Semin Neurol. 2010. 30: 311-9
18. Sierra-Hidalgo F, Penas-Prado M, Martín-Gil L, HerrerosRodríguez J, Hilario-Barrio A, Ruiz-Morales J. Cerebral infarctions and leptomeningeal carcinomatosis. Rev Neurol. 2009. 49: 156
19. Taillibert S, Chamberlain MC.editors. Leptomeningeal metastasis. Handbook of Clinical Neurology. Elsevier BV; 2018. 149: 169-204
20. Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: Review and update on management. Cancer. 2018. 124: 21-35
21. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: Experience with 90 patients. Cancer. 1982. 49: 759-72
22. Winkler F. Pathogenesis and biology. Handb Clin Neurol. 2018. 149: 43-56
23. Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020. 383: 944-57