- Department of Neurosurgery, Fujita Health University Hospital, Toyoake, Japan
- Department of Diagnostic Pathology, Fujita Health University Hospital, Toyoake, Japan
Correspondence Address:
Joji Inamasu
Department of Diagnostic Pathology, Fujita Health University Hospital, Toyoake, Japan
DOI:10.4103/2152-7806.185775
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ganaha T, Inamasu J, Oheda M, Hasegawa M, Hirose Y, Abe M. Subarachnoid hemorrhage caused by an undifferentiated sarcoma of the sellar region. Surg Neurol Int 07-Jul-2016;7:
How to cite this URL: Ganaha T, Inamasu J, Oheda M, Hasegawa M, Hirose Y, Abe M. Subarachnoid hemorrhage caused by an undifferentiated sarcoma of the sellar region. Surg Neurol Int 07-Jul-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/subarachnoid-hemorrhage-caused-undifferentiated-sarcoma-sellar-region/
Abstract
Background:It is rare for patients with pituitary apoplexy to exhibit concomitant subarachnoid hemorrhage (SAH). Only a handful of patients with pituitary apoplexy have developed such hemorrhagic complications, and histopathological examination revealed pituitary adenoma as the cause of SAH.
Case Report:A previously healthy 35-year-old woman was brought to our institution after complaining of severe headache and left monocular blindness. Brain computed tomography showed a diffuse SAH with a central low density. Subsequently, the brain magnetic resonance imaging revealed an intrasellar mass with heterogeneous contrast enhancement. The patient was presumptively diagnosed with SAH secondary to hemorrhagic pituitary adenoma and underwent transcranial surgery to remove both the tumor and subarachnoid clot. A histological evaluation of the surgical specimen revealed malignant cells with strong predilection for vascular invasion. Following immunohistochemical evaluation, the tumor was negative for the majority of tumor markers and was positive only for vimentin and p53; thus, a diagnosis of undifferentiated sarcoma was established.
Conclusions:This case was informative in the respect that tumors other than pituitary adenoma should be included in the differential diagnosis of patients with pituitary apoplexy.
Keywords: Pituitary apoplexy, sella turcica, subarachnoid hemorrhage, undifferentiated sarcoma
INTRODUCTION
Pituitary apoplexy is a rare cause of hemorrhage in the pituitary gland. In most circumstances, bleeding does not expand beyond the pituitary gland due to the presence of the surrounding membranes, such as the arachnoid membrane and diaphragma sellae, which function as a barrier.[
CASE REPORT
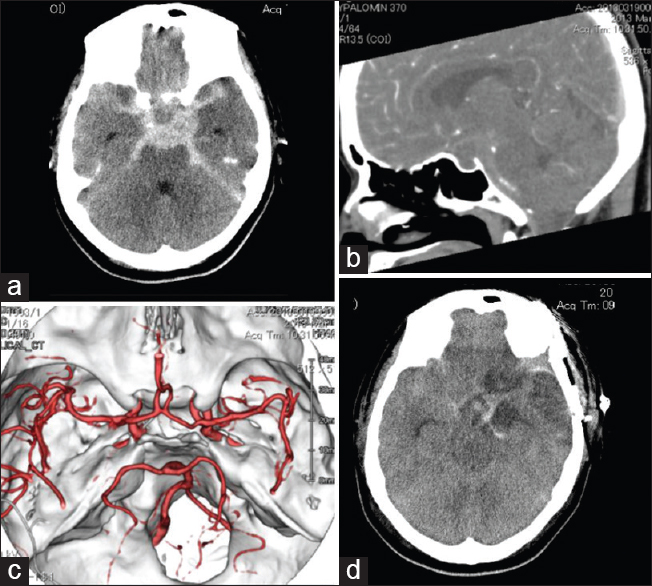
A previously healthy 35-year-old woman who had no history of radiation exposure was brought to our institution after complaining of severe headache and left monocular blindness. Her consciousness level on arrival was E1V4M5 on Glasgow Coma Scale. Brain computed tomography (CT) revealed a diffuse SAH with a central low density [
Figure 1
Computed tomography of the brain showing subarachnoid chemorrhage with a central low density (a). No substantial sellar enlargement is observed with a sagittal reconstructed view (b). Brain computed tomography angiography showing the absence of a ruptured aneurysm (c). Computed tomography obtained 7 days postoperatively showing extensive cerebral infarction due to vasospasm (d)
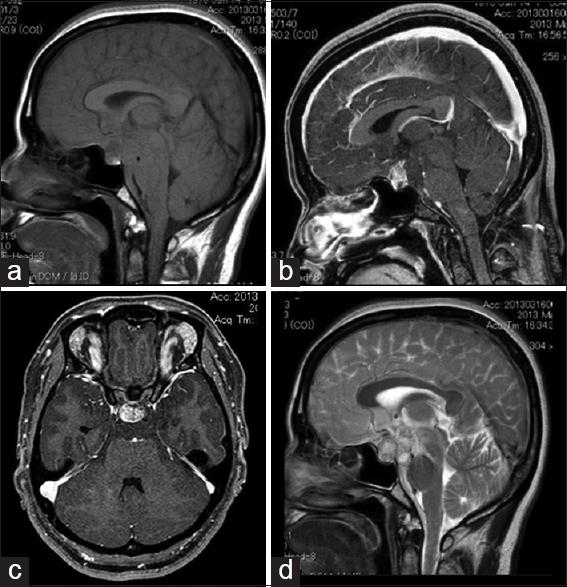
Figure 2
Magnetic resonance imaging showing an intrasellar mass which is depicted as low-intensity on a nonenhanced T1-weighted image (a). With gadolinium, the mass exhibits strong enhancement (b, sagittal view; c, axial view). On a T2-weighted image, the mass is depicted as heterogeneous high intensity, and a dense subarachnoid clot was also observed in the prepontine cistern (d)
For removal of the tumor, we chose a transcranial approach via frontotemporal craniotomy rather than a transsphenoidal approach, with a hope of reducing the amount of SAH in the basal cistern, and the patient was brought to the operation room following administration of intravenous hydrocortisone. During surgery, the tumor was found to be soft and was easily aspirated. However, the tumor could only be partially removed due to profuse bleeding from the tumor and swelling of the surrounding brain tissue as a result of SAH. The interface between the tumor and the diaphragma sellae could not be fully inspected, and the position of the pituitary stalk could not be confirmed. Postoperative management included administration of intravenous vasopressin, hydrocortisone, and oral levothyroxine, and the patient was maintained normovolemic/normotensive to prevent delayed vasospasm. Despite transient mild improvement in the consciousness level following surgery, the patient sustained a large cortical infarction secondary to the vasospasm [
Pathological examination
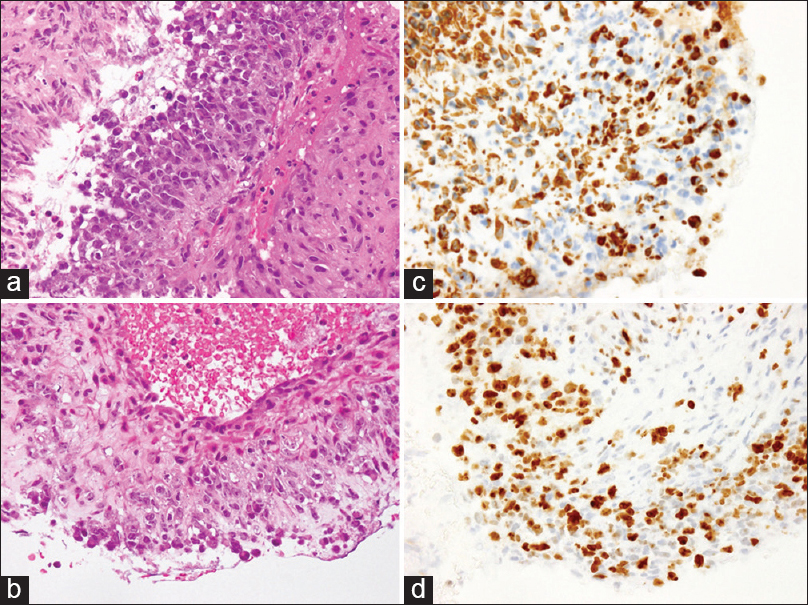
On hematoxylin and eosin staining, the tumor cells were found to be densely proliferated either in a medullary or in a trabecular pattern. Densely arranged tumor cells with large, irregular nuclei and many mitotic figures were observed [
Figure 3
On H and E staining, the tumor cells are seen to have proliferated densely, either in a medullary or trabecular pattern, and densely arranged tumor cells with large, irregular nuclei and many mitotic figures are observed (a). In another area, tumor invasion to the venous wall is observed (b). Although an extensive immunohistochemical study was performed to identify the histological origin of the tumor, it is positive only for vimentin (c) and p53. The MIB-1 labeling index is 63.6%, indicating potent proliferative activity of the tumor (d)
DISCUSSION
Although the precise pathological mechanism of pituitary apoplexy has not yet been fully elucidated, the great majority of patients have underlying pituitary adenomas which often remain silent. Pituitary adenoma cells have a higher energy demand/consumption than the surrounding brain tissue, and episodes of hypoxia and/or hypoglycemia are known to result in necrotic cell death and subsequent bleeding from the degenerated adenoma cells.[
The present case is unique in the respect that SAH was caused by an undifferentiated sarcoma and not by a pituitary adenoma. Sarcomas in the sellar region in the absence of a history of radiation exposure are very rare, with <10 cases reported in the literature excluding hemangiopericytoma.[
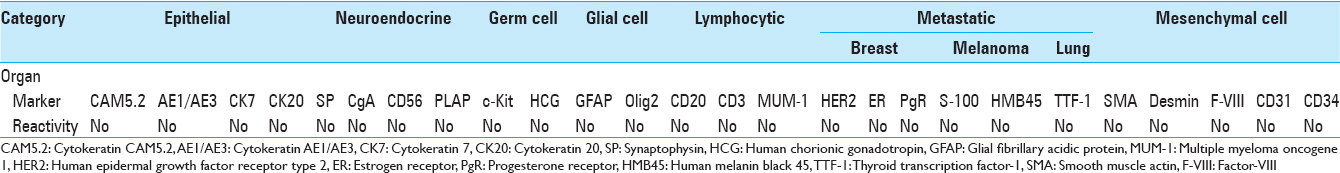
Specifying the histological origin of the tumor was difficult due to lack of immunoreactivity to tumor markers barring vimentin and p53 [
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alpert TE, Hahn SS, Chung CT, Bogart JA, Hodge CJ, Montgomery C. Successful treatment of spindle cell sarcoma of the sella turcica.Case report. J Neurosurg. 2002. 97: 438-40
2. Arita K, Sugiyama K, Tominaga A, Yamasaki F. Intrasellar rhabdomyosarcoma: Case report. Neurosurgery. 2001. 48: 677-80
3. Capatina C, Inder W, Karavitaki N, Wass JA. Management of endocrine disease: Pituitary tumour apoplexy. Eur J Endocrinol. 2015. 172: R179-90
4. Glezer A, Bronstein MD. Pituitary apoplexy: Pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015. 59: 259-64
5. Inamasu J, Hori S, Sekine K, Aikawa N. Pituitary apoplexy without ocular/visual symptoms. Am J Emerg Med. 2001. 19: 88-90
6. Jalali R, Srinivas C, Nadkarni TD, Rajasekharan P. Suprasellar haemangiopericytoma - Challenges in diagnosis and treatment. Acta Neurochir (Wien). 2008. 150: 67-71
7. Manoranjan B, Syro LV, Scheithauer BW, Ortiz LD, Horvath E, Salehi F. Undifferentiated sarcoma of the sellar region. Endocr Pathol. 2011. 22: 159-64
8. Nakahara K, Oka H, Utsuki S, Iida H, Kurita M, Mochizuki T. Pituitary apoplexy manifesting as diffuse subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 2006. 46: 594-7
9. Oldfield EH, Merrill MJ. Apoplexy of pituitary adenomas: The perfect storm. J Neurosurg. 2015. 122: 1444-9
10. Rhoton AL. The sellar region. Neurosurgery. 2002. 51: S335-74
11. Sareen P, Chhabra L, Trivedi N. Primary undifferentiated spindle-cell sarcoma of sella turcica: Successful treatment with adjuvant temozolomide. BMJ Case Rep 2013. 2013. p.
12. Satyarthee GD, Mahapatra AK. Pituitary apoplexy in a child presenting with massive subarachnoid and intraventricular hemorrhage. J Clin Neurosci. 2005. 12: 94-6
13. Singh TD, Valizadeh N, Meyer FB, Atkinson JL, Erickson D, Rabinstein AA. Management and outcomes of pituitary apoplexy. J Neurosurg. 2015. 122: 1450-7
14. Tomita H, Urui S, Kokunai T, Tamaki N. A case of metastatic tumor of the pituitary gland presenting as a subarachnoid hemorrhage. No Shinkei Geka. 2000. 28: 1117-20
15. Turgut M, Ozsunar Y, Basak S, Güney E, Kir E, Meteoglu I. Pituitary apoplexy: An overview of 186 cases published during the last century. Acta Neurochir (Wien). 2010. 152: 749-61
16. Wohaibi MA, Russell NA, Ferayan AA, Awada A, Jumah MA, Omojola M. Pituitary apoplexy presenting as massive subarachnoid hemorrhage. J Neurol Neurosurg Psychiatry. 2000. 69: 700-1