- Department of Spine Services, Indian Spinal Injuries Centre, New Delhi,
- Department of Intern, Seth GSMC and KEM Hospital, Mumbai, Maharashtra, India.
Correspondence Address:
Nandan Marathe, Department of Spine Services, Indian Spinal Injuries Centre, New Delhi, India.
DOI:10.25259/SNI_909_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abhinandan Reddy Mallepally1,2, Amrit Gantaguru1, Nandan Marathe1, Tarush Rustagi1, Alhad Mulkalwar2, Kalidutta Das1. Subaxial cervical Castleman’s disease: A rare cause of myelopathy. 08-Nov-2021;12:552
How to cite this URL: Abhinandan Reddy Mallepally1,2, Amrit Gantaguru1, Nandan Marathe1, Tarush Rustagi1, Alhad Mulkalwar2, Kalidutta Das1. Subaxial cervical Castleman’s disease: A rare cause of myelopathy. 08-Nov-2021;12:552. Available from: https://surgicalneurologyint.com/surgicalint-articles/11222/
Abstract
Background: Castleman’s disease (CD) is a rare lymphoproliferative disease of unknown origin which rarely affects the spine. Here, we present CD involving a lytic, destructive C3 lesion with extension into the spinal canal contributing to upper cervical cord compression. Notably, the lesion mimicked other primary bone lesions, metastatic tumors, and/or lymphoma.
Case Description: A 52-year-old male presented with progressive quadriparesis (i.e. weakness, instability of gait) and loss of dexterity in both hands over 2 weeks. The MRI, X-ray, and CT scans revealed a destructive lytic lesion involving the C3 vertebral body (i.e. including both anterior and posterior elements). The patient underwent a C3 total and C4 partial laminectomy followed by a C2-C4/5 instrumented fusion (i.e. included C2 pedicle screws/laminar screws, and C4/C5 lateral mass fixation). Histopathology showed a lymphoproliferative disorder with follicles of different sizes, central abnormal germinal structures, and a Mantle zone (i.e. expanded germinal centre with concentric layering with an “onionskin” appearance). These findings were all consistent with the diagnosis of CD (i.e. hyaline-vascular type).
Conclusion: CD, a rare lymphoproliferative disease of unknown origin rarely affects the spine. Here, we presented a 52-year-old male with a C3 lytic lesion resulting in C3/4 cord compression that favorably responded to a C3/4 laminectomy with posterior instrumented fusion.
Keywords: Castleman’s, Cervical spine, Lymph node, Myelopathy
INTRODUCTION
Castleman’s disease (CD) is a rare lymphoproliferative disease of unknown origin that generally affects lymph nodes, rarely the chest, neck, abdomen, pelvis, axilla, sometimes the lung, parotid gland, pancreas, and spine.[
The differential diagnoses for CD include - infection, malignancy, autoimmune, and/or collagen vascular disease. Eight other cases of spinal CD [
CASE DESCRIPTION
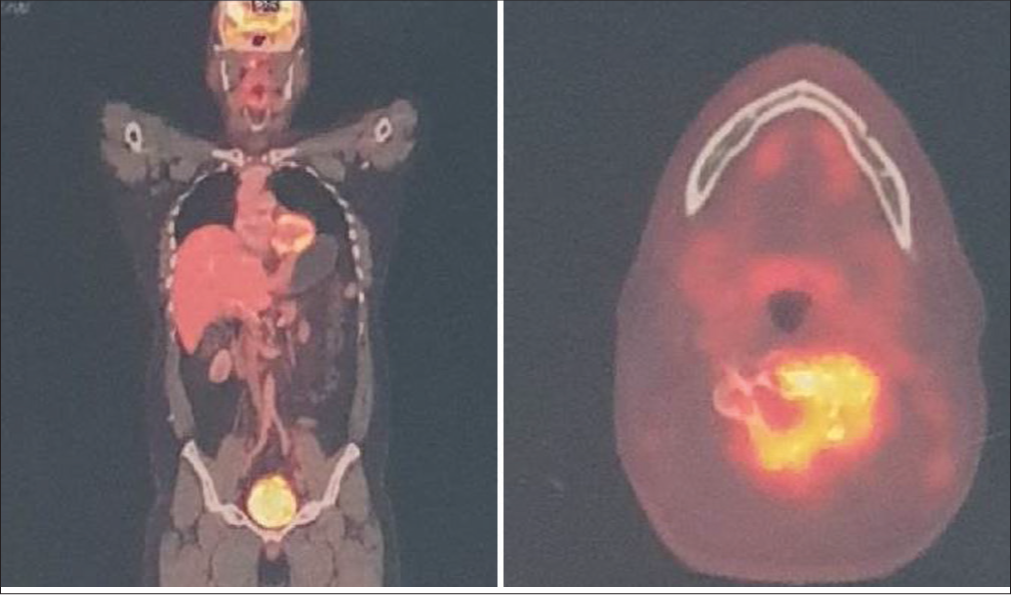
A 52-year-old male presented with 2 weeks of a progressive quadriparesis (i.e. loss of dexterity in both hands and lower extremity weakness) and urinary incontinence. He also had cervical lymphadenopathy (i.e. anterior/posterior and post-auricular lymph nodes). On exam, he had 4/5 weakness in both lower extremities, diffuse lower extremity hyper-reflexia with bilateral Babinski responses, and a T2 sensory level (mJOA-10/18).
Radiological studies
The X-rays and CT scan revealed a destructive lytic C2-C4 lesion focused at the C3 level vertebral body (i.e. destructive lytic lesion of anterior/posterior C3, the C2 spinous process, and anterior C4 vertebral body) [
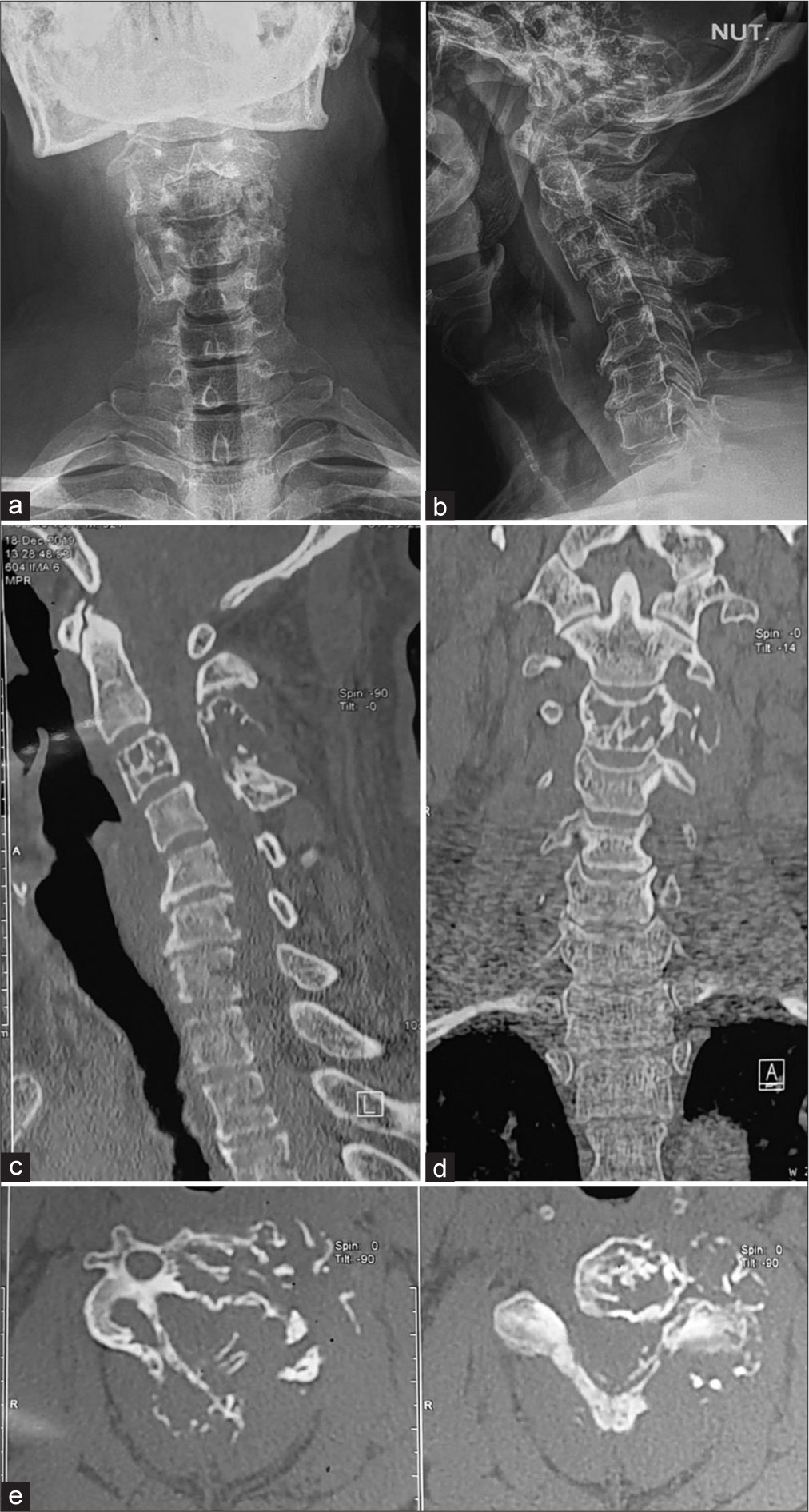
Figure 1:
Anteroposterior (a) and Lateral (b) radiographsshowinglyticexpansilelesion in thepoosteriorelemnts of C3 spinous process with cervical spine in mild kyphosis. Computer tomography images Sagittal (c), Coronal (d) andAxial images (e) showing lytic expansile lesion involving C3 vertebral body and posterior elements more onto the left side.
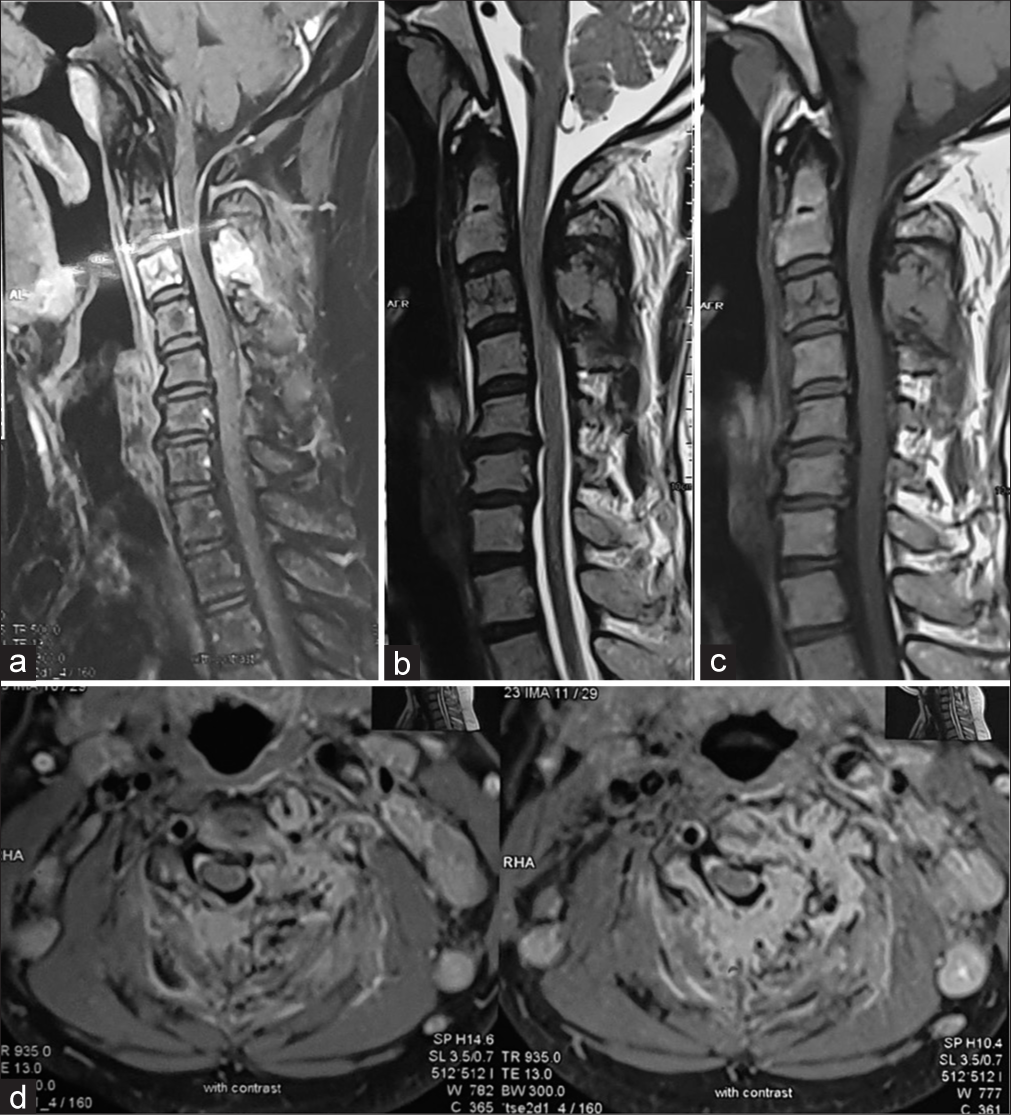
Figure 2:
Sagittal section of STIR (a), T2 weighted (b) and T1 weighted (c) images showing high signal intensity on Stir images in C3 vertebral body and posterior elements and hypointense signal on T1 weighted images. Cord compression due to epidural mass effect can be appreciated from posterior aspect. (d) Axial T2 weighted images at C3 vertebral body level showing destruction of Spinous process and left sided lamina and lateral mass with epidural tissue compressing and displacing the spinal cord.
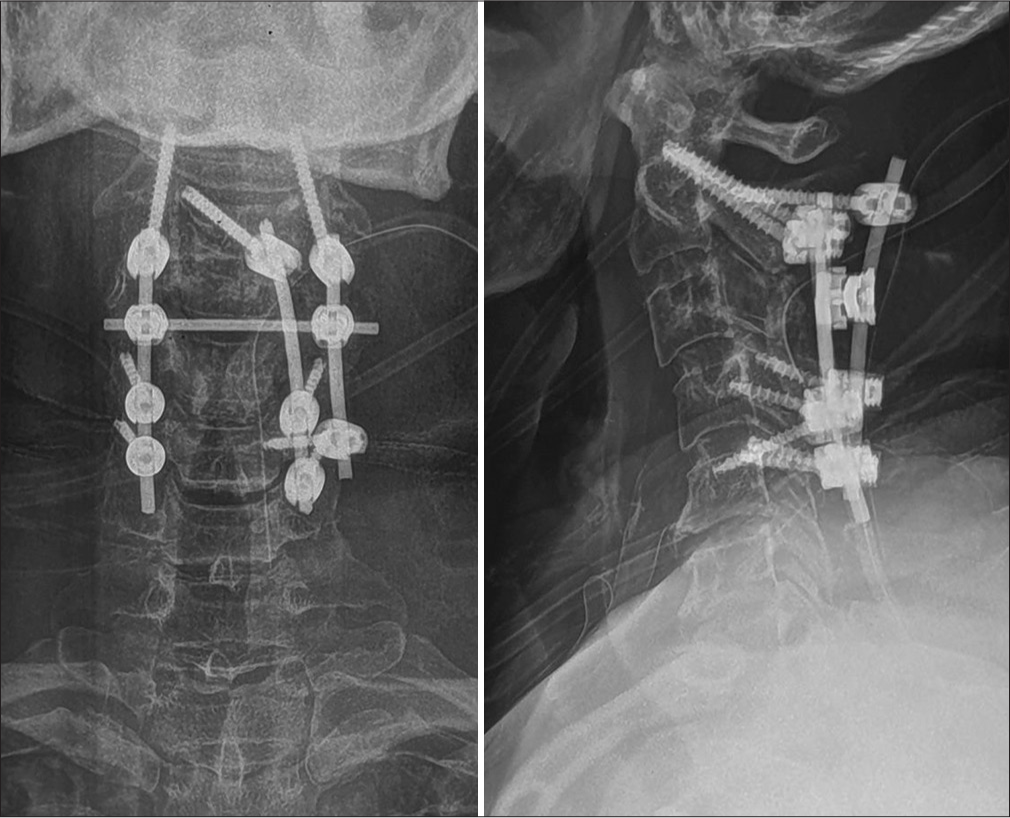
Surgery
In view of a Spinal Instability Neoplastic Score of 14, the evidence of spinal cord compression and likely instability, the patient underwent a C3-4/laminectomy with C2-4/5 posterior fusion. At surgery, the posterior cervical paraspinal musculature infiltrated by tumor and had to be removed. A novel “three rod construct” fusion was performed that included C2 pedicle screws, laminar screws, a C4 left pedicle screw with lateral mass fixation of C4 and C5 [
Histopathology
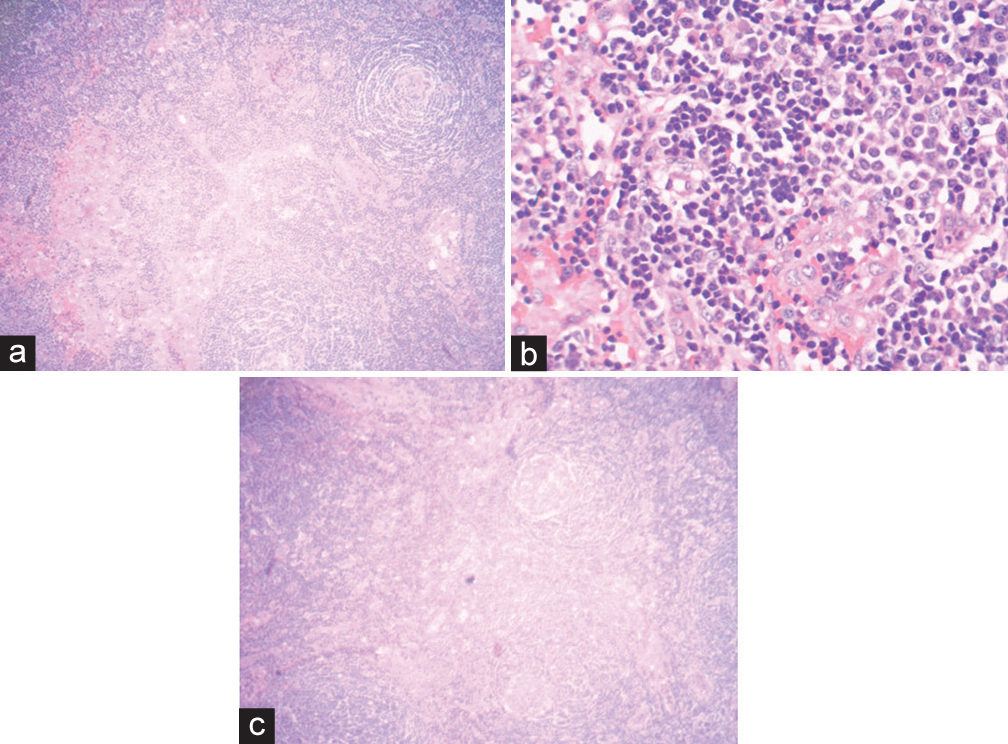
Histopathology was consistent with a lymphoproliferative disorder (i.e. with follicles of different size with abnormal germinal structures at their centres with hyaline-vascular or “burnt-out” appearances) [
Figure 5:
Haematoxylin and Eosin photomicrographs demonstrating (a) a germinal centre with hyaline deposits and penetrated by sclerotic blood vessels and typical concentric rings of small lymphocytes in an onion skin composition. (b) Dense lymphoid infiltrate with hyperplastic lymphoid follicles with small, hyalinised germinal centres, mantle cell hyperplasia. (c) Low power view of the tissue showing vascular and lymphoid proliferation hyalinised blood vessels penetrating radially into the follicles.
Adjuvant therapy
The patient was started on methylprednisolone for the lymphadenopathy, and diagnosis of CD. This was followed by radiotherapy (total dose 3960cGy in 22 fractions).
Follow-up 1 year later
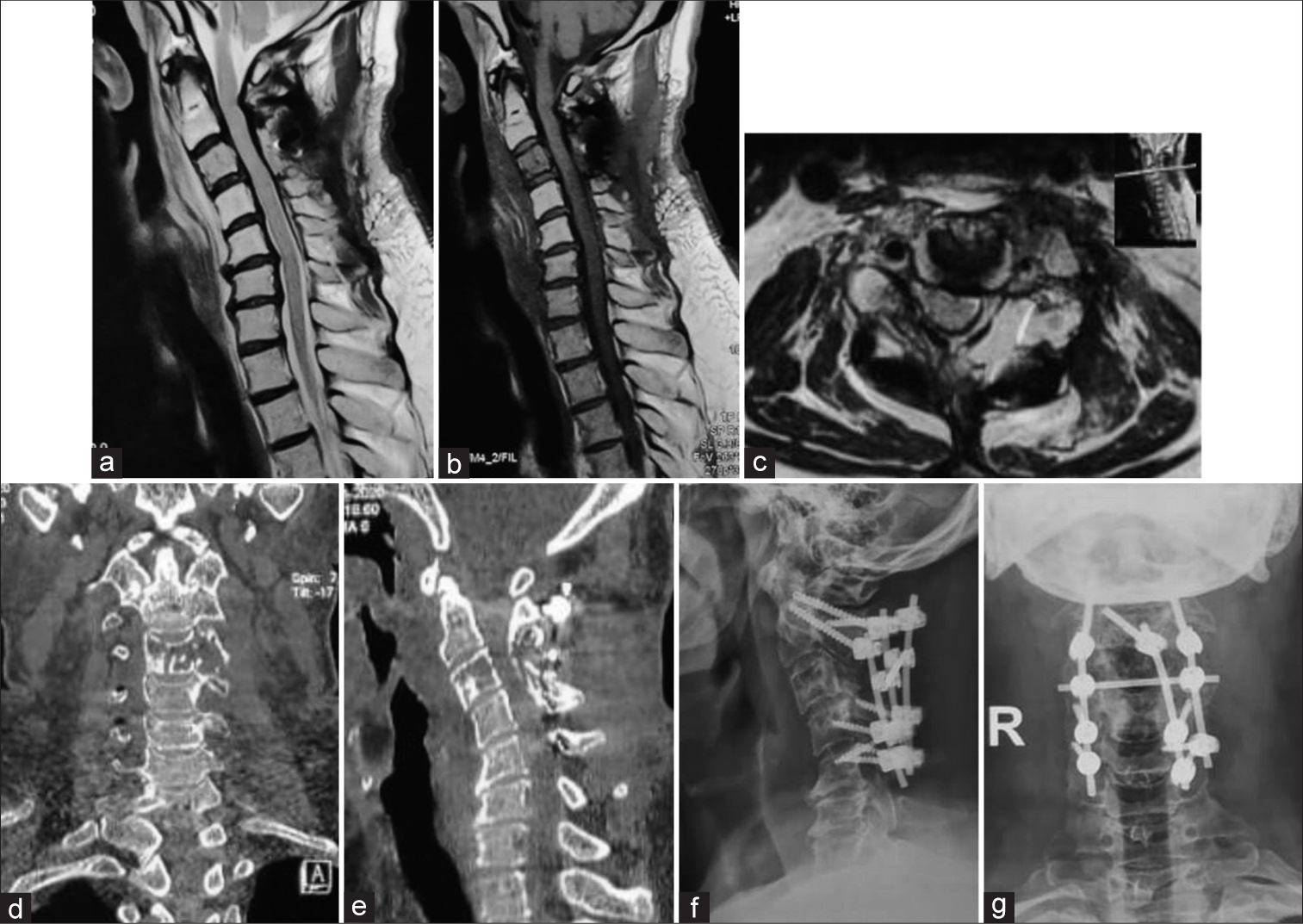
At the last follow-up 1 year later, patient remained asymptomatic without evidence of lesion progression [
Figure 6:
Follow-up T2 weighted (a) and T1 weighted (b) sagittal MR images showing adequate decompression of cord and axial MRI (c) showing adequate resolution of tumour. Coronal (d) and sagittal (e) images showing resolution of tumorous mass and lateral (f) and anteroposterior (g) radiographs showing no signs of implant failure.
DISCUSSION
CD is a rare lymphoproliferative disorder that is histologically categorized as a benign, hyaline-vascular type, representing approximately 80–90% of cases; the remaining 10–20% are aggressive multifocal form - plasma cell type lesions.[
The treatment modalities for CD include surgical excision for single lymph node involvement. Complete surgical resection generally offers complete cure in those with a localized variant, while those with more extensive diffuse unresectable lesions typically warrant additional chemotherapy, immunotherapy, and/or radiation therapy. Histopathology of CD may resemble other conditions such as lymphoma, autoimmune disorders like rheumatoid arthritis, Sjögren’s syndrome, monoclonal gammopathy, and acquired immunodeficiency syndrome. A definitive diagnosis of CD requires pathological-histological confirmation (i.e. biopsy or tumor resection). Those patients with the localized form may be treated with local resection or radiation therapy[
CONCLUSION
CD rarely involves the spine. Here, it presented involving the C2-C4 levels (i.e. focused at C3) with cord compression warranting a C3/4 laminectomy and C2-C4/5 posterior fusion.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation
Conflicts of interest
There are no conflicts of interest.
References
1. Arai K, Mukae J, Akiyama M, Kumagai J, Yamamoto K. Successful treatment of pure red cell aplasia complicated by multicentric Castleman disease with prednisolone. Rinsho Ketsueki. 2020. 61: 122-7
2. Castleman B, Iverson L, Menendez VP. Localized mediastinal lymph-node hyperplasia resembling thymoma. Cancer. 1956. 9: 822-30
3. Finn MA, Schmidt MH. Castleman disease of the spine mimicking a nerve sheath tumor. Case report. J Neurosurg Spine. 2007. 6: 455-9
4. Gianaris PG, Leestma JE, Cerullo LJ, Butler A. Castleman’s disease manifesting in the central nervous system: Case report with immunological studies. Neurosurgery. 1989. 24: 608-12
5. Gupta A, Kumar L, Karak A, Thulkar S, Rastogi R, Bhatti S. Multicentric hyaline vascular type Castleman disease presenting as an epidural mass causing paraplegia: A case report. Clin Lymphoma Myeloma. 2009. 9: 250-3
6. McMillan R, Goodwin CR, Hsu W, Pendleton J, McCarthy E, Wolinsky JP. Castleman disease of the sacral spine. Clin Neurol Neurosurg. 2012. 114: 287-9
7. Min GJ, Jeon YW, Park SS, Park S, Shin SH, Yahng SA. The clinical, laboratory, and radiologic improvement due to siltuximab treatment in idiopathic multicentric Castleman’s disease. Korean J Intern Med. 2020. 36: 424-32
8. Severson GS, Harrington DS, Weisenburger DD, McComb RD, Casey JH, Gelber BR. Castleman’s disease of the leptomeninges, Report of three cases. J Neurosurg. 1988. 69: 283-6
9. van Rhee F, Voorhees P, Dispenzieri A, Fosså A, Srkalovic G, Ide M. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018. 132: 2115-24
10. Weisenburger DD, DeGowin RL, Gibson P, Armitage JO. Remission of giant lymph node hyperplasia with anemia after radiotherapy. Cancer. 1979. 44: 457-62