- Department of Neurosurgery, Faculty of Medicine, The University of Tokyo,
- Department of Neurosurgery, Tokyo Metropolitan Neurological Hospital, Tokyo, Japan,
- Department of Surgery, Division of Neurotrauma, Hospital Tengku Ampuan Rahimah, Selangor Darul Ehsan,
- Department of Medicine, Melaka Manipal Medical College, Manipal University, Melaka, Malaysia.
Correspondence Address:
S. Muruga Subramaniam
Department of Neurosurgery, Faculty of Medicine, The University of Tokyo,
DOI:10.25259/SNI_516_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: S. Muruga Subramaniam, Kazuhiko Ishii, Chen Jui Sheng, Hirofumi Nakatomi, Keisuke Takai, Nobuhito Saito. Successful surgical strategy for ventral thoracic spinal perimedullary spinal arteriovenous fistulas: Case report. 20-Dec-2019;10:251
How to cite this URL: S. Muruga Subramaniam, Kazuhiko Ishii, Chen Jui Sheng, Hirofumi Nakatomi, Keisuke Takai, Nobuhito Saito. Successful surgical strategy for ventral thoracic spinal perimedullary spinal arteriovenous fistulas: Case report. 20-Dec-2019;10:251. Available from: https://surgicalneurologyint.com/surgicalint-articles/9811/
Abstract
Background: Spinal arteriovenous fistulas (AVFs) are vascular lesions that often pose significant surgical challenges. This is particularly true for those located close to the anterior spinal artery. Here, we analyzed the surgical options for treating an anterior perimedullary AVF (pAVFs).
Case Description: A 66-year-old male with the right lower extremity weakness was diagnosed with a spinal dural AVF at the L1 level. It was initially treated with open surgery followed by CyberKnife radiosurgery at another institution. Five years later, he presented with a persistent pAVF fistula now involving the T11 level; the major feeder originated on the left at the T7–T8 level (e.g., involving a left-sided “duplicated” anterior spinal artery). Utilizing a three-dimensional (3D) computer tomography (CT) guided approach; he underwent a left-sided posterolateral T10–T12 laminectomy, sufficient to allow for 30–40° of anterior spinal cord rotation. This was performed under neurophysiological monitoring without any significant changes. Surgery included indocyanine green video angiography, temporary feeder clipping, and complete occlusion of the AVF, followed by complete clipping/resection as confirmed on postoperative magnetic resonance imaging.
Conclusion: Utilizing a 3D CT image, a ventral pulmonary arteriovenous malformation was excised utilizing a left-sided posterolateral approach allowing for 30–40° of cord rotation.
Keywords: Expert computer tomography, Indocyanine green, Perimedullary arteriovenous fistulas, Posterolateral surgical approach, Three-dimensional image
INTRODUCTION
Spinal perimedullary arteriovenous fistulas (pAVFs) are relatively rare lesions (incidence of 8–19%) that commonly involve the conus medullaris or cauda equina.[
CASE DESCRIPTION
A 66-year-old male patient presented with the right lower extremity weakness. On magnetic resonance (MR) and 3D-CT, he has had a spinal dural AVF at the L1 level. Six months after onset of the symptoms, a left L1 feeder was clipped. However, postoperatively, the patient’s clinical condition worsened due to a cerebrospinal fluid leak that required secondary corrective surgery (e.g., 1 month after the first surgery). Notably, the routine follow-up angiogram 6 months later, demonstrated a persistent pAVF.
Five years later
Five years later, the patient presented with progressive muscle atrophy/paraparesis and urinary incontinence. The ventral thoracic spinal pAVF was now visualized at the T11 level on the MR. Angiographically, it was supplied by the anterior spinal artery [
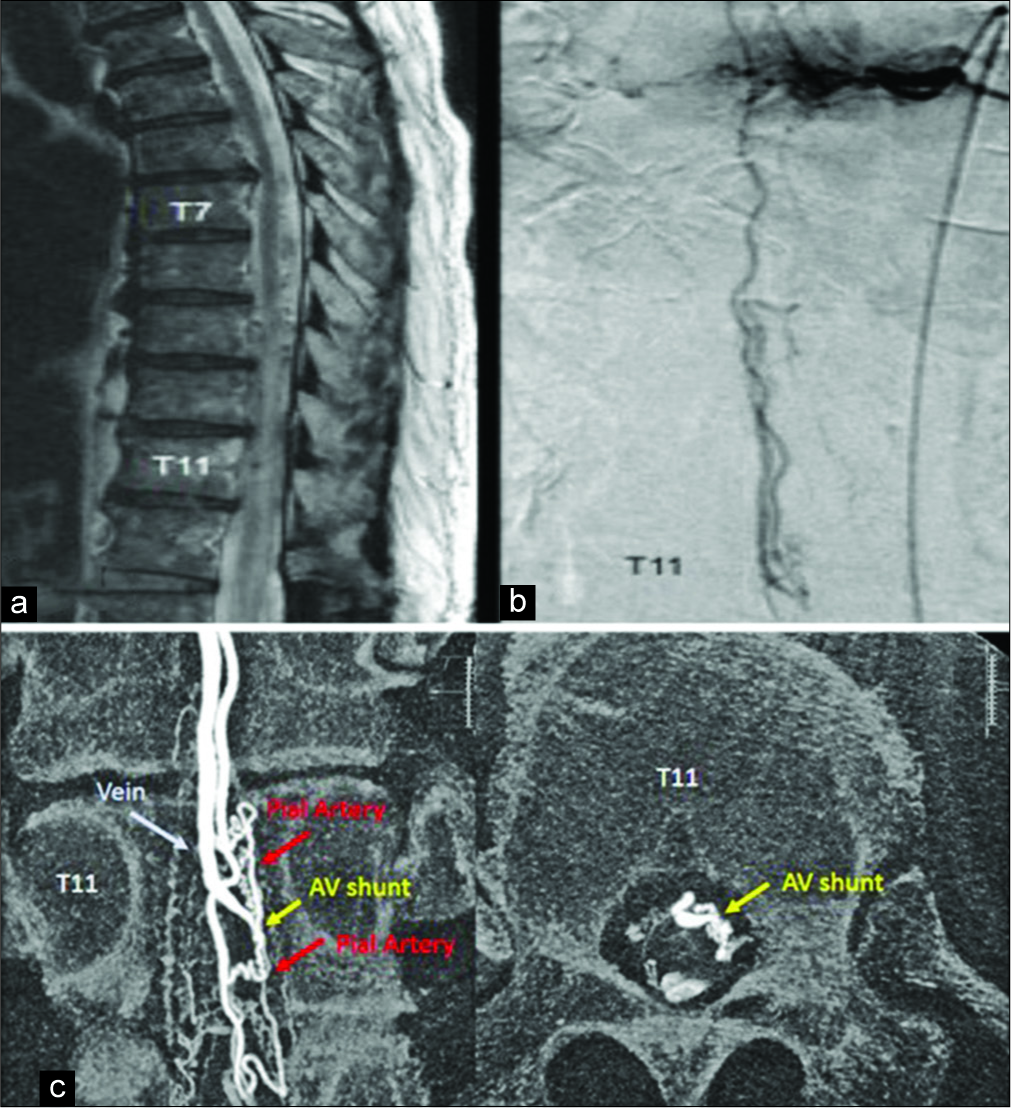
Figure 1:
(a) Preoperative sagittal T2-weighted magnetic resonance images showing abnormal vessels as a flow void around the spinal cord and intramedullary hyperintensity. (b) T11 angiographic imaging demonstrating the relationship between anterior spinal artery, draining vein, pial artery, and arteriovenous (AV) shunt. (c) Preoperative high-resolution angiography showing anterior spinal artery, pial artery, AV shunt, and draining vein.
Surgical strategy
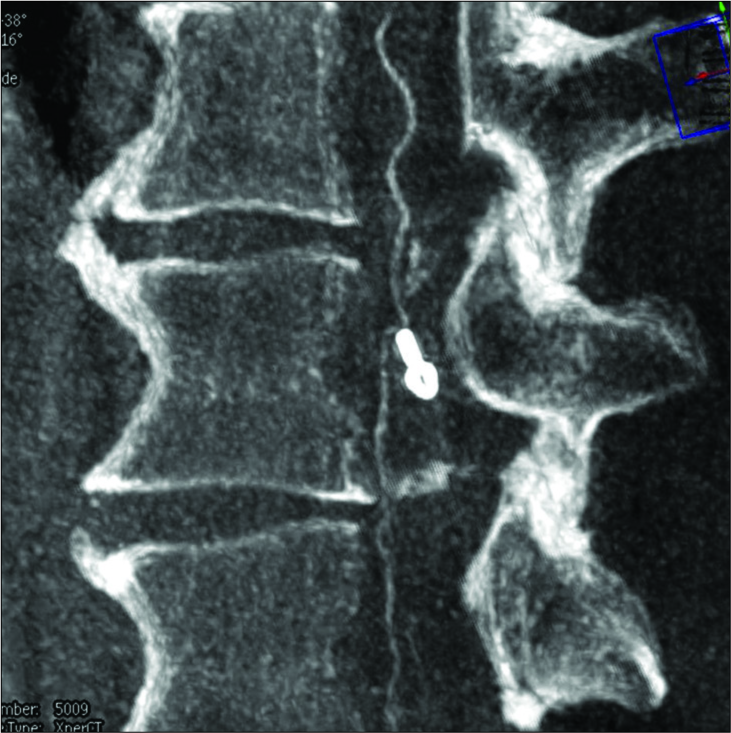
Based on the 3D-CT, a posterolateral left-sided T10–T12 laminectomy was performed that could allow for 30–60° of cord rotation to access the ventral AVF [
Notably, motor evoked potentials (MEPs) and somatosensory evoked potentials did not change during and after cord rotation/manipulation. There was a direct arteriovenous connection between the anterior spinal artery (ASA) and a thin-walled abnormal vein, and a duplication of the ASA for the segment harboring the fistula [
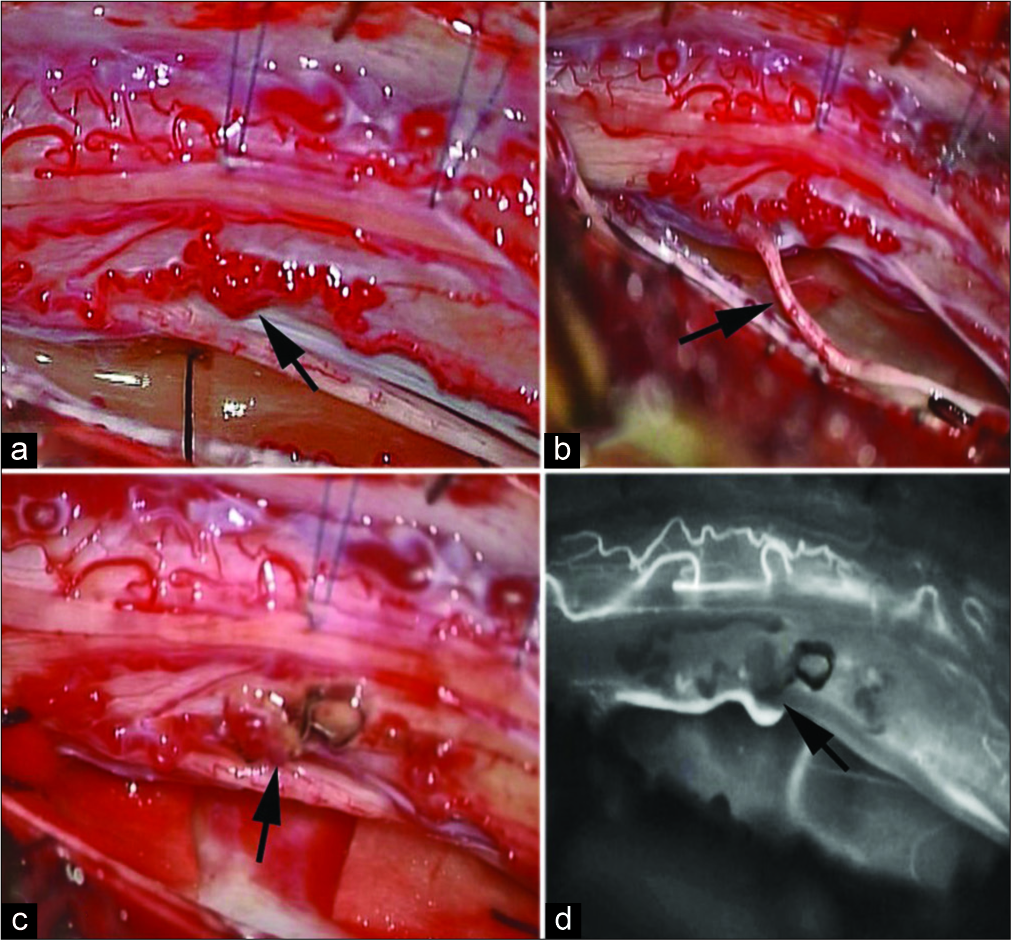
Figure 3:
(a) Intraoperative arrow image of cord rotation with shunting point and draining vein visible. (b) Intraoperative arrow image showing perimedullary arteriovenous fistula (pAVF) on the ventrolateral surface of the rotated cord with the ipsilateral dentate ligament. (c) Arrow image showing temporary clipping of proximal and distal anterior spinal artery (ASA) feeding the pAVF. (d) Arrow image of complete resection of pAVF and indocyanine green confirmation of the flow in the ASA.
Postoperative MR imaging
The postoperative MR did not show any new or significant intramedullary cord changes. Six months later, it continued to show complete occlusion/resection of the pAVF [
Histopathology
The histopathological examination confirmed abnormal vascular structures corresponding to the pAVF. The Verhoeff Van Gieson stain showed smooth muscle and elastic fibers inside the blood vessels that had variable wall thickening/ caliber that correlated with a pAVF [
Figure 5:
Histopathology: Verhoeff Van Gieson stain, arrowhead shows abnormal vascular structures with smooth muscle and elastic fibers inside the blood vessel walls, the latest with variable thickens and vessel caliber, irregular intimal hyperplasia and hyalinized walls. Arrow shows the possible shunting areas.
DISCUSSION
Type of AVF
In this case, MR imaging and angiography demonstrated a Type IV pAVF, subtype I pAVFs. The success rate for permanent pAVF occlusion was previously reported as 98% for open surgery, but a lesser 10–75% for endovascular embolization.[
CONCLUSION
Utilizing preoperative 3D CT planning to estimate the necessary/extent of cord rotation, safe exposure, and excision of a ventrolateral spinal pAVFs was successfully accomplished at the T11 level.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barrow DL, Colohan AR, Dawson R. Intradural perimedullary arteriovenous fistulas (type IV spinal cord arteriovenous malformations). J Neurosurg. 1994. 81: 221-9
2. Djindjian M, Djindjian R, Rey A, Hurth M, Houdart R. Intradural extramedullary spinal arterio-venous malformations fed by the anterior spinal artery. Surg Neurol. 1977. 8: 85-93
3. Hurst RW.editors. Vascular Disorders of the Spine and Spinal Cord. Philadelphia, PA: Lippicott Williams and Wilkins; 2002. p. 1825-54
4. Mourier KL, Gobin YP, George B, Lot G, Merland JJ. Intradural perimedullary arteriovenous fistulae: Results of surgical and endovascular treatment in a series of 35 cases. Neurosurgery. 1993. 32: 885-91
5. Onda K, Yoshida Y, Watanabe K, Arai H, Okada H, Terada T. High cervical arteriovenous fistulas fed by dural and spinal arteries and draining into a single medullary vein: Report of 3 cases. J Neurosurg Spine. 2014. 20: 256-64
6. Porras M, Juvela S. Spinal cord and spinal dural arteriovenous fistulas case report. J Neurosurg. 1993. 32: 885-91
7. Sherif C, Gruber A, Bavinzski G, Standhardt H, Widhalm G, Gibson D. Long-term outcome of a multidisciplinary concept of spinal dural arteriovenous fistulae treatment. Neuroradiology. 2008. 50: 67-74
8. Steinmetz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ. Outcome after the treatment of spinal dural arteriovenous fistulae: A contemporary single-institution series and meta-analysis. Neurosurgery. 2004. 55: 77-87