- Department of Neurosurgery, Gunma University Graduate School of Medicine, Mebashi,
- Department of Neurosurgery, Subaru Health Insurance Society Ota Memorial Hospital, Ota, Gunma, Japan.
Correspondence Address:
Takahiko Nakazawa, Department of Neurosurgery, Gunma University Graduate School of Medicine, Mebashi, Gunma, Japan.
DOI:10.25259/SNI_95_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takahiko Nakazawa1, Masanori Aihara1, Hiroyuki Mizuno2, Rei Yamaguchi1, Yuhei Yoshimoto1. Superior sagittal sinus dural arteriovenous fistula with changes in angiographic findings associated with contiguous parasagittal meningioma: A case report. 23-Jun-2022;13:275
How to cite this URL: Takahiko Nakazawa1, Masanori Aihara1, Hiroyuki Mizuno2, Rei Yamaguchi1, Yuhei Yoshimoto1. Superior sagittal sinus dural arteriovenous fistula with changes in angiographic findings associated with contiguous parasagittal meningioma: A case report. 23-Jun-2022;13:275. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11682
Abstract
Background: Meningioma and dural arteriovenous fistula (dAVF) located at the same site are rare. The present case demonstrated the transformation of tumor feeding vessels into the pial feeder of the dAVF over time, which may help to elucidate the pathogenesis of tumor-associated dAVF.
Case Description: A 71-year-old man presented with convulsion. Magnetic resonance (MR) imaging showed a right parasagittal sinus meningioma invading the superior sagittal sinus (SSS). Bilateral external carotid angiography showed dAVF at the SSS, near the site of tumor invasion. The right internal carotid angiography showed tumor staining from the anterior cerebral artery with intra-tumor arteriovenous shunting, with stagnation of tumor blood flow, suggesting impairment of perfusion to the SSS. Four years after the initial diagnosis, the patient was admitted to hospital with status epilepticus, and MR imaging showed an enlarged tumor. Carotid angiography revealed transformation of the tumor feeders to the pial feeder of the dAVF. The findings of shunting to the SSS had intensified, and stenosis had occurred in the posterior third of the SSS. The venous return showed retrograde flow anteriorly to the SSS. The patient underwent endovascular embolization and tumor resection. The shunt had disappeared.
Conclusion: This report supports the proposal that impaired venous return is an important factor in the shunt occurrence of dAVF. Neurosurgeons should consider that cases of meningioma invading the venous sinuses may be complicated by dAVF and changes may occur over time.
Keywords: Dural arteriovenous fistula, Meningioma, Venous pressure
INTRODUCTION
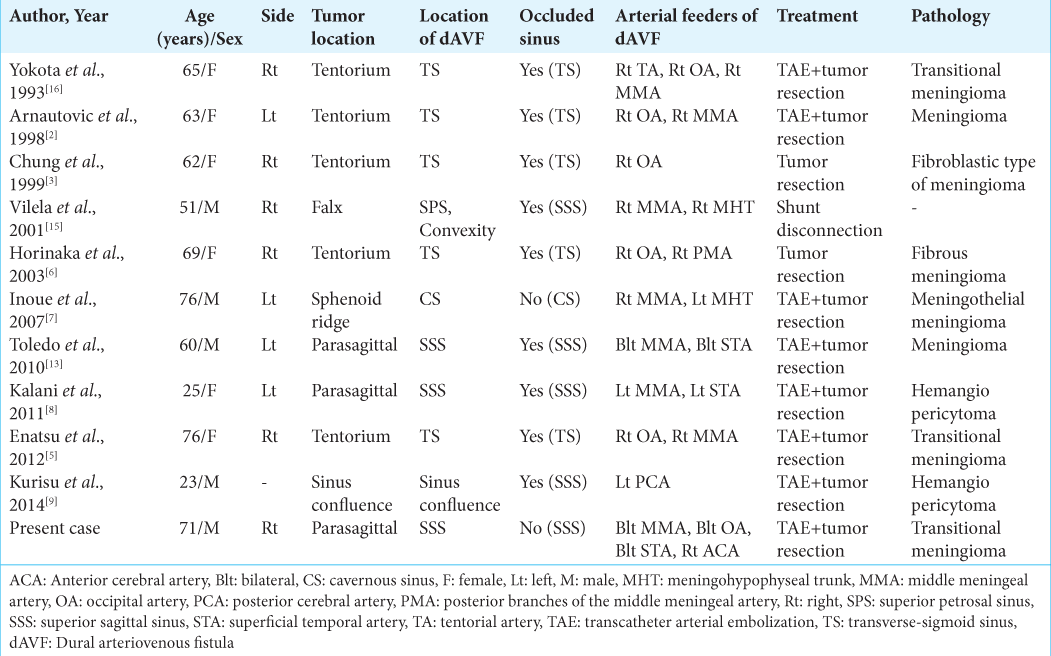
The etiology of dural arteriovenous fistula (dAVF) is still unclear, but may include congenital factors and acquired factors such as venous sinus hypertension caused by obstruction of the venous sinuses due to trauma, surgical invasion, infection, or tumor.[
We experienced a rare case, in which cerebral angiography showed that the tumor feeding vessels of a parasagittal meningioma with SSS invasion were transformed into the pial feeder for a dAVF that developed in the same region, with acute aggravation of the vessels. This observation may suggest a link to the pathogenesis of tumor-associated dAVF.
CASE DESCRIPTION
A 71-year-old man presented with convulsive symptoms. Computed tomography (CT) showed a 3-cm isodense mass in the right parasagittal region. T1-weighted magnetic resonance (MR) imaging showed prominent enhancement with dural tail sign after gadolinium-diethylenetriaminepenta-acid acid administration, suggestive of meningioma [
After the initial diagnosis, the seizures were controlled and the neurological findings detected no abnormalities. The patient did not wish to receive treatment and was discharged for follow-up. Four years after the first diagnosis, he was hospitalized with status epilepticus causing intractable convulsive aggravation, so deep sedation/ventilator control was established for 5 days with propofol and midazolam. On admission, CT showed small subcortical hemorrhage in the left frontal lobe [
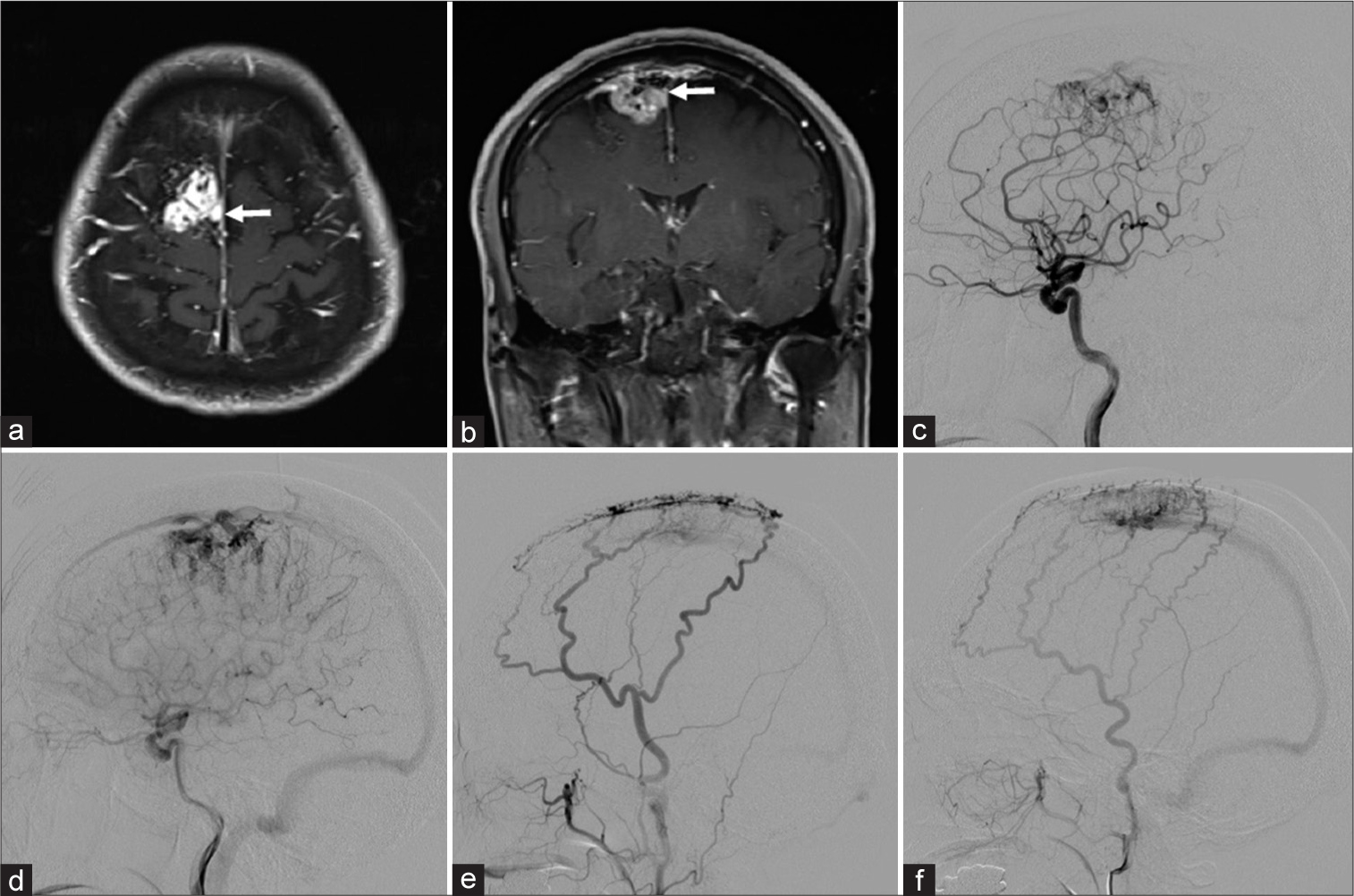
Figure 1:
Parasagittal meningioma. Axial (a) and coronal (b) T1-weighted magnetic resonance images showing an enhanced tumor in the right parasagittal region that extends toward the superior sagittal sinus (SSS) (white arrow). (c) Lateral right internal carotid angiogram, arterial phase, showing a tumor blush supplied by the anterior cerebral artery (ACA). (d) Lateral right internal carotid angiogram, capillary phase, showing stasis of tumor blood flow. (e) Lateral right external carotid angiogram showing that both the middle meningeal artery (MMA) and superficial temporal artery (STA) supply the dural arteriovenous fistula (dAVF). (f) Lateral left external carotid angiogram showing that both the MMA and STA supply the dAVF.
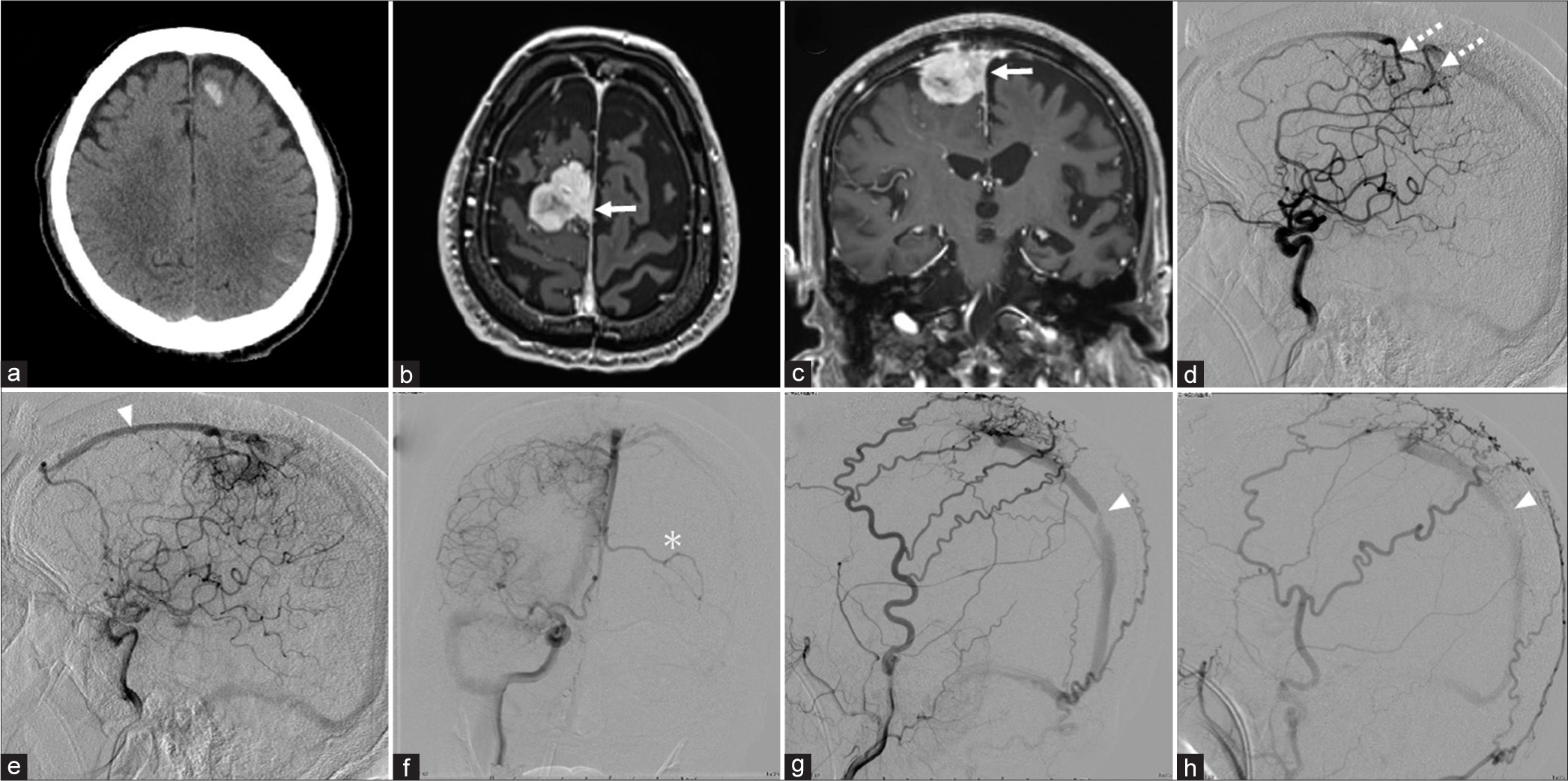
Figure 2:
(a) Computed tomography scan showing left frontal subcortical hemorrhage. Axial (b) and coronal (c) T1-weighted magnetic resonance images showing a homogeneous enhanced growing tumor in the right parasagittal region that extends toward the superior sagittal sinus (SSS) (white arrow). (d) Lateral right internal carotid angiogram, arterial phase, showing a pial AVF fed by the ACA (white dotted arrow). (e) Lateral right internal carotid angiogram, capillary phase, showing retrograde blood flow anteriorly in the SSS (white arrowhead). (f) Anteroposterior right internal carotid angiogram showing cortical venous reflux in the left frontal cortex (white asterisk). Lateral right (g) and left (h) external carotid angiograms showing that the MMA, STA, and occipital artery supply the dAVF and stenosis of the posterior third of the SSS (white arrowhead).
We hypothesized that the impaired cerebral venous drainage due to stenosis of the outflow tract of the SSS dAVF was the cause of the prolonged disturbance of consciousness. To reduce the shunt blood flow, ligation of bilateral STAs and embolization of the bilateral MMAs and OAs with platinum coils were performed. Post embolization angiography showed a significant decrease in shunt blood flow from the MMAs, OAs, and STAs. However, the tumor staining and pial shunt from the ACA, and the left frontal lobe cortical venous reflux persisted [
Video 1
Postoperative MR imaging showed partial removal of the tumor except for the part inside the SSS [
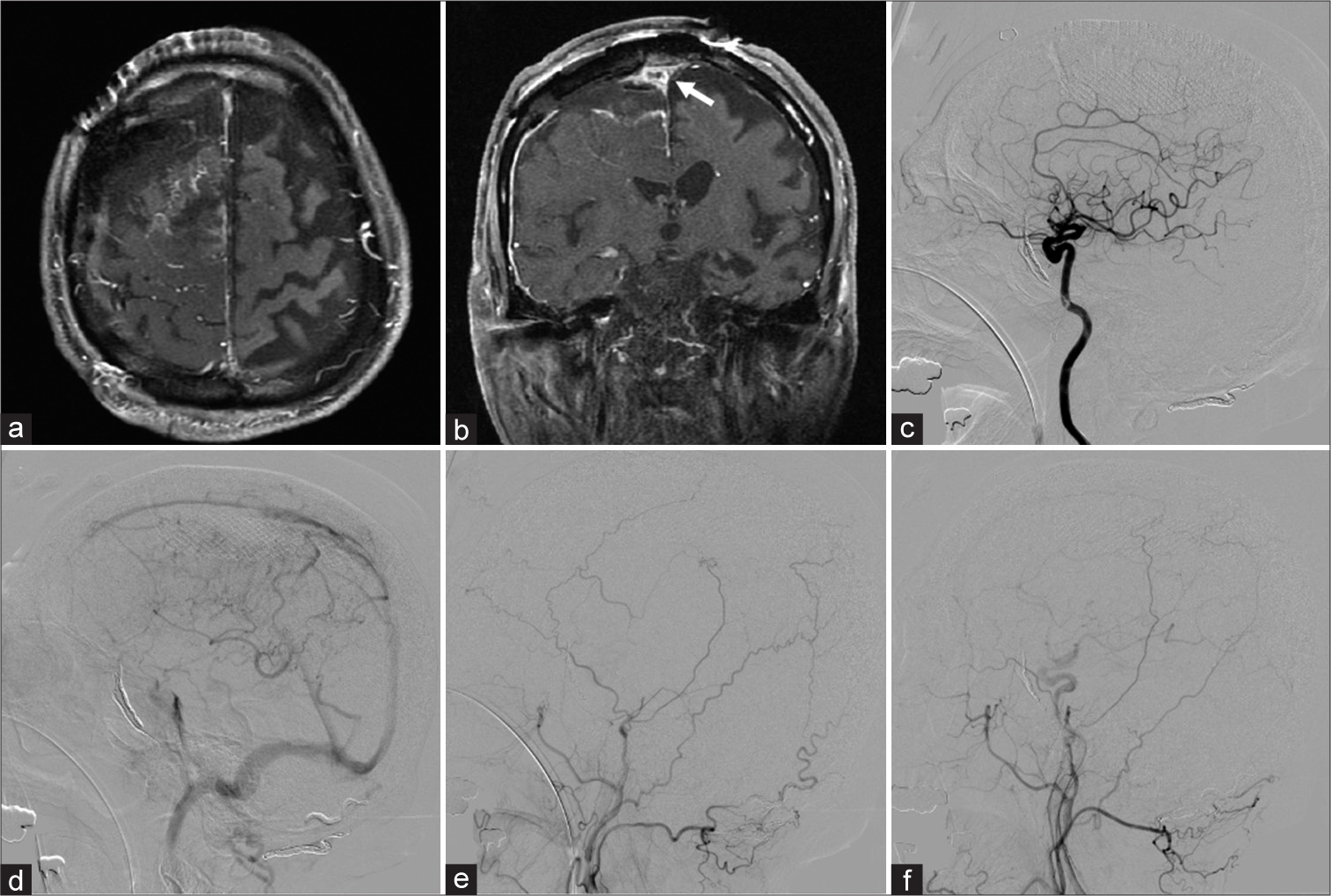
Figure 4:
Postoperative axial (a) and coronal (b) T1-weighted magnetic resonance images confirming gross total resection of the tumor except for the superior sagittal sinus (SSS) invasion (white arrow). (c) Postoperative lateral right internal carotid angiogram, arterial phase, demonstrating disappearance of a pial AVF fed by the ACA. (d) Lateral right internal carotid angiogram, venous phase, showing antegrade flow of the SSS. Lateral right (e) and left (f) external carotid angiograms demonstrating disappearance of the dAVF.
DISCUSSION
The rapid worsening of this case was apparently caused by stenosis of the posterior third of the SSS, which is the outflow route of the dAVF with increased shunt blood flow. In addition, cerebral angiography confirmed transformation of the tumor feeding vessels into the pial feeder of the dAVF.
dAVF is a rare disease, accounting for 10–15% of all intracranial arteriovenous malformations. However, the causes of dAVF are still unknown, but are thought to be associated with a history of head trauma or craniotomy, tumor, infection, or cerebral venous sinus embolism.[
The mechanism of secondary dAVF in intracranial tumors has not been clarified. The proposed mechanisms by which meningiomas may be associated with dAVFs include direct increase in venous pressure due to invasion or compression of the venous sinuses by the tumor, indirect increase in venous pressure due to brain edema around the tumor, increase in venous pressure due to meningioma with abundant blood flow, hypercoagulability of the tumor itself, or induction of angiogenic substances such as hypoxia-inducible factor-1 and vascular endothelial growth factor by the meningioma.[
Changes in the angiographic findings were also noteworthy. At the time of the first seizure, carotid angiography showed that the blood flow of the ACA, which is the feeding vessel of the tumor, stagnated due to the increased venous pressure of the SSS. However, 4 years later, the findings of flow stagnation in the tumor feeders were improved and predominantly appeared as a pial feeder of the dAVF in the SSS. At the time of the first seizure, flow stagnation in the tumor feeder may reflect relatively early changes in intracranial blood flow due to the venous hypertension associated with dAVF. Then, we speculate that the persistent stagnation of the tumor blood flow from the ACA may have triggered the formation of a pial shunt from the ACA to the SSS dAVF. We also speculate that the persistent increase in venous pressure caused stenosis of the SSS, leading to acute exacerbation of the dAVF symptoms. Therefore, this case may provide valuable data for elucidating the mechanism of secondary dAVF associated with tumors, based on our imaging of the changes in the shunting of the dAVF.
No definitive treatment policy has been established for cases of dAVF associated with meningioma. Some cases of dAVF spontaneously disappeared after only meningioma removal,[
CONCLUSION
This case report may support the proposal that impaired venous return is an important factor in the shunting of dAVF. Neurosurgeons should consider that cases of meningioma invading the venous sinuses may be complicated by dAVF and changes may occur over time.
Videos available on:
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahn JY, Lee BH, Cho YJ, Joo JY, Lee KS. Dural arteriovenous fistula associated with meningioma: Spontaneous disappearance after tumor removal. Neurol Med Chir (Tokyo). 2003. 43: 308-11
2. Arnautovic KI, Al-Mefty O, Angtuaco E, Phares LJ. Dural arteriovenous malformations of the transverse/sigmoid sinus acquired from dominant sinus occlusion by a tumor: Report of two cases. Neurosurgery. 1998. 42: 383-8
3. Chung YG, Lee KC, Lee HK, Lee NJ. Tentorial meningioma encroaching the transverse sinuses and sigmoid sinus junction area associated with dural arteriovenous fistulous malformation: A case report. J Korean Med Sci. 1999. 14: 465-8
4. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995. 194: 671-80
5. Enatsu R, Asahi M, Matsumoto M, Hirai O. Meningioma-related dural arteriovenous fistula fed via a vascular tumor bed: A case report and literature review. Clin Neurol Neurosurg. 2012. 114: 1010-3
6. Horinaka N, Nonaka Y, Nakayama T, Mori K, Wada R, Maeda M. Dural arteriovenous fistula of the transverse sinus with concomitant ipsilateral meningioma. Acta Neurochir (Wien). 2003. 145: 501-4
7. Inoue T, Nishimura S, Hayashi N, Numagami Y, Tomita T, Nishijima M. Cavernous sinus dural arteriovenous fistula associated with the development of sphenoidal ridge meningioma--case report. Neurol Med Chir (Tokyo). 2007. 47: 317-21
8. Kalani MY, Martirosyan NL, Eschbacher JM, Nakaji P, Albuquerque FC, Spetzler RF. Large hemangiopericytoma associated with arteriovenous malformations and dural arteriovenous fistulae. World Neurosurg. 2011. 76: 592.e7-10
9. Kurisu K, Motegi H, Osanai T, Kobayashi H, Terasaka S, Houkin K. Regression of dural arteriovenous fistulae after venous flow reconstructive surgery in a case with hemangiopericytoma at the confluence of sinuses. Case Rep Neurol. 2014. 6: 207-12
10. Manzo M, D’Urso PI. Convexity meningioma associated with noncontiguous dural arteriovenous fistula. Surg Neurol Int. 2020. 11: 127
11. Reynolds MR, Lanzino G, Zipfel GJ. Intracranial dural arteriovenous fistulae. Stroke. 2017. 48: 1424-31
12. Terada T, Higashida RT, Halbach VV, Dowd CF, Tsuura M, Komai N. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994. 80: 884-9
13. Toledo MM, Wilson TJ, Dashti S, McDougall CG, Spetzler RF. Dural arteriovenous fistula associated with superior sagittal sinus occlusion secondary to invasion by a parafalcine meningioma: Case report. Neurosurgery. 2010. 67: 205-7
14. Vellimana AK, Daniels DJ, Shah MN, Zipfel GJ, Lanzino G. Dural arteriovenous fistulas associated with benign meningeal tumors. Acta Neurochir (Wien). 2014. 156: 535-44
15. Vilela P, Willinsky R, terBrugge K. Dural arteriovenous fistula associated with neoplastic dural sinus thrombosis: two cases. Neuroradiology. 2001. 43: 816-20
16. Yokota M, Tani E, Maeda Y, Yamaura I. Meningioma in sigmoid sinus groove associated with dural arteriovenous malformation: Case report. Neurosurgery. 1993. 33: 316-9
17. Zhou LF, Chen L, Song DL, Gu YX, Leng B. Dural arteriovenous fistula of the sphenobasilar sinus with concomitant meningioma: Case report and review of the literature. Neurosurg Rev. 2007. 30: 269-74