- Department of Neurosurgery, Tokyo General Hospital, Tokyo, Japan,
- Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq,
- Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States
- Department of Neurosurgery, University of Cincinnati, Cincinnati, Ohio, United States.
Correspondence Address:
Awfa Aktham Abdulateef, Department of Neurosurgery, Tokyo General Hospital, Tokyo, Japan.
DOI:10.25259/SNI_251_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Awfa Aktham Abdulateef1, Shuhei Morita1, Mustafa Ismail2, Mayur Sharma3, Samer S. Hoz4, Shinichi Numazawa1, Yasunobu Ito1, Sadayoshi Watanabe1, Kentaro Mori1. Supraorbital keyhole approach for paraclinoid aneurysms clipping: A case series with literature review. 12-May-2023;14:167
How to cite this URL: Awfa Aktham Abdulateef1, Shuhei Morita1, Mustafa Ismail2, Mayur Sharma3, Samer S. Hoz4, Shinichi Numazawa1, Yasunobu Ito1, Sadayoshi Watanabe1, Kentaro Mori1. Supraorbital keyhole approach for paraclinoid aneurysms clipping: A case series with literature review. 12-May-2023;14:167. Available from: https://surgicalneurologyint.com/surgicalint-articles/12323/
Abstract
Background: Paraclinoid aneurysms (PcAs) are challenging aneurysms due to the complexity of their relation to the surrounding bony and neurovascular structures. Although over the past decade, their management strategy has shifted from transcranial to endovascular approaches; here, we try to revolve around a subcategory to which minimal invasive supraorbital keyhole (SOK) surgery is feasible depending on specific radiological criteria with a literature review.
Methods: A group of unruptured PcAs was managed surgically, with a subset that was clipped through the SOK approach. They were selected by preoperative simulation images using 3D computed tomography (CT) angiography (CTA). We also conducted an extensive literature review based on a database available on PubMed and Google Scholar, the yielded cases from the literature review plus our cases were analyzed according to six parameters including their size, location, dome direction, need for clinoidectomy and proximal cervical control, and surgical outcome.
Results: From February 2009 to August 2022, 49 cases of unruptured PcAs were managed by clipping, and of these, four cases were clipped by the SOK approach, in addition, four cases were yielded through the literature review. The sizes of the PcAs ranged from 3 to 8 mm. Their location fluctuated from anterior to the superomedial wall and their domes pointed superiorly except for one which points posteriorly. Six of eight cases required anterior clinoidectomy, the outcome was uneventful.
Conclusion: A subset of unruptured PcAs are amenable to SOK with criteria such as unruptured small aneurysm (
Keywords: Ophthalmic aneurysm, Paraclinoid aneurysm, Supraorbital keyhole approach
INTRODUCTION
The paraclinoid aneurysm (PcA) was described first by Nutik.[
PcA clipping mostly requires anterior clinoidectomy, optic canal opening, and resection of the dural ring, therefore standard or modified pterional approach including the Dolenc skull base technique is preferable; however, a subset of PcA can be clipped through minimal invasive keyhole mini-craniotomy.[
The supraorbital keyhole (SOK) surgery provides adequate aneurysmal access while minimizing trauma to the surrounding structures, including the skin, bone, dura, and, most importantly, the brain,[
We intend to propose parameters for selecting the SOK approach in clipping PcA aneurysms by discussing our four PcA aneurysms that were clipped using SOK surgery and reviewing the literature.
MATERIALS AND METHODS
Present cases
From February 2009 to August 2022, a group of patients with unruptured PcAs were managed surgically, and a subset of them was clipped through SOK with maximum craniotomy size (27~30 mm) at Tokyo General Hospital and National Defense Medical College. This study was approved by the Local Institutional Review Boards of Tokyo General Hospital and National Defense Medical College. Patient consent was not required due to the study’s retrospective nature from medical records, and patients’ identities were not included in the study.
Preoperative simulation
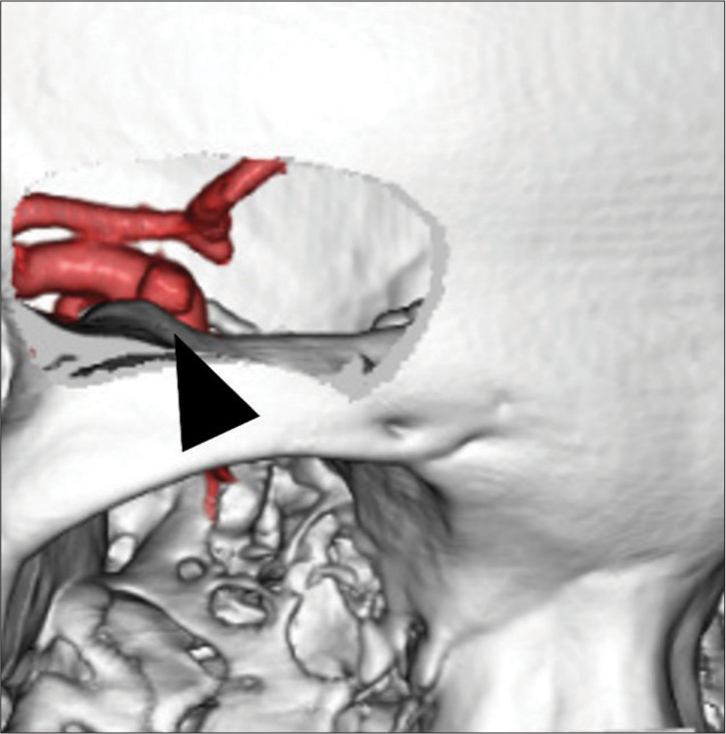
Preoperative planning was based on 3D computed tomography angiography (CTA) with a constant medium. Data were transferred to a workstation (Ziostation 2.4; Ziosoft, Inc., Tokyo, Japan). Optimal keyhole size and location were simulated. The anatomical relationship between PcA and the anterior clinoid process (ACP) was also checked [
Surgical technique
Our surgical technique has been previously published.[
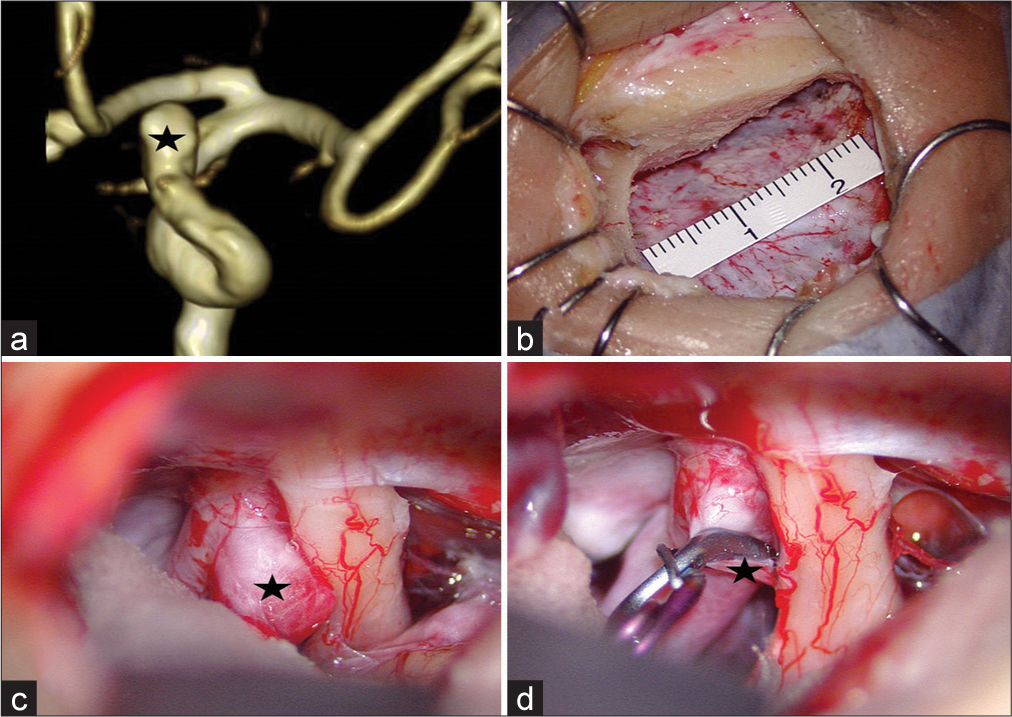
Figure 2:
Preoperative image and intraoperative views of the left paraclinoid aneurysm (black star). (a) The preoperative magnetic resonance angiography shows a superiorly projecting anterior wall-type paraclinoid aneurysm. (b) Intraoperative view of Supraorbital keyhole mini-craniotomy craniotomy size. (c) The paraclinoid aneurysm was attached and compressed the left optic nerve but apart from the anterior clinoid process. (d) Intraoperative view post aneurysmal clipping.
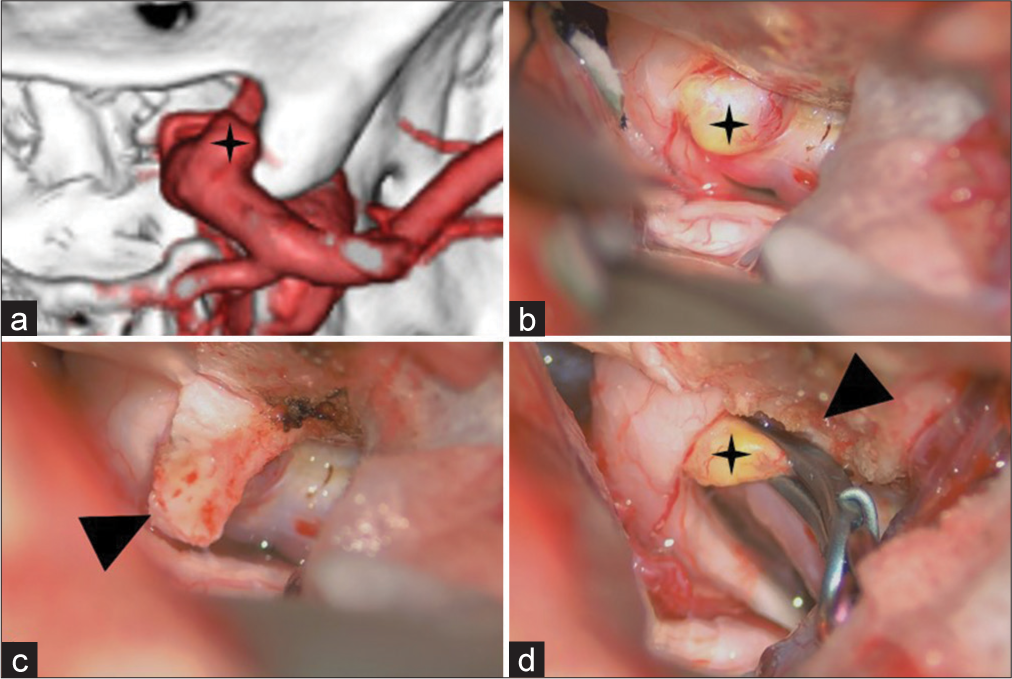
Figure 3:
Preoperative image and intraoperative views of the right paraclinoid aneurysm (black star). (a) The preoperative 3D computed tomography angiography shows the paraclinoid aneurysm attaching to the anterior clinoid process (ACP). (b) Intraoperatively, the aneurysmal dome is attached to the right optic nerve and the ACP. (c) The dura mater flap (arrowhead) was peeled from the ACP and covered over the aneurysm for protection during the drilling of the ACP. (d) Post partial removal of ACP (arrowhead) and aneurysmal clipping.
Postoperative imaging
On the day after the operation, CT, 3D CTA, and magnetic resonance including diffusion-weighted images were performed to determine the completeness of the clipping and identified any hemorrhagic and ischemic complications. If no clinical or radiological abnormalities were confirmed, the patient was discharged a few days after the operation.
Literature review
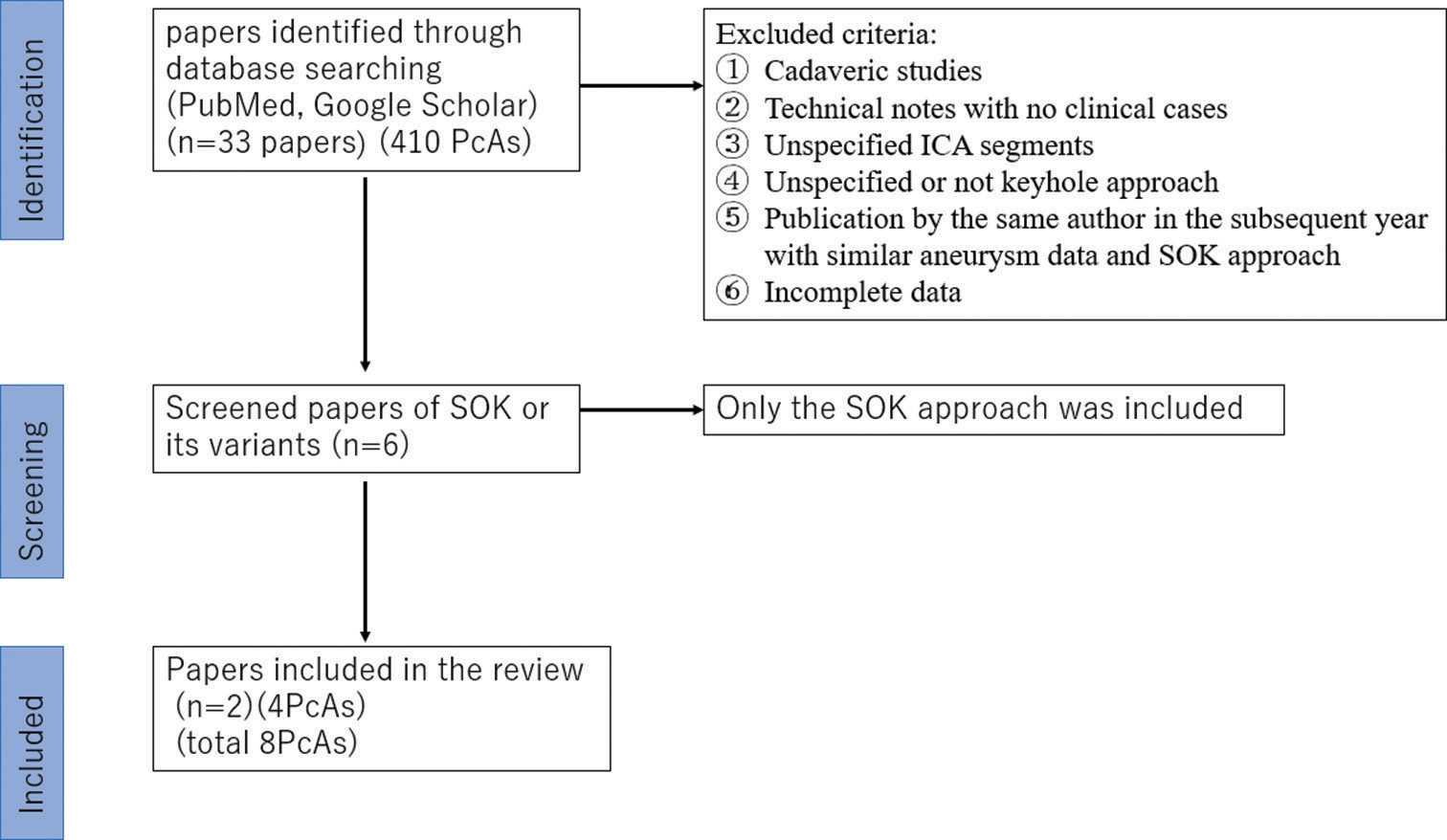
The review of the literature was performed on two different online medical databases (PubMed, and Google scholar), using search terms such as “supraorbital keyhole,” “pterional keyhole,” “paraclinoid aneurysm” “superior hypophyseal aneurysm,” “carotid cave aneurysm,” and “juxtadural ring aneurysm” (being SOK and pterional keyhole considered as falling under this definition) (All Fields), combined with the Boolean operators “OR” and “AND.” The last search was conducted on August 31, 2022, and went back as far as data were available. The search strategy is summarized in
RESULTS
Present cases
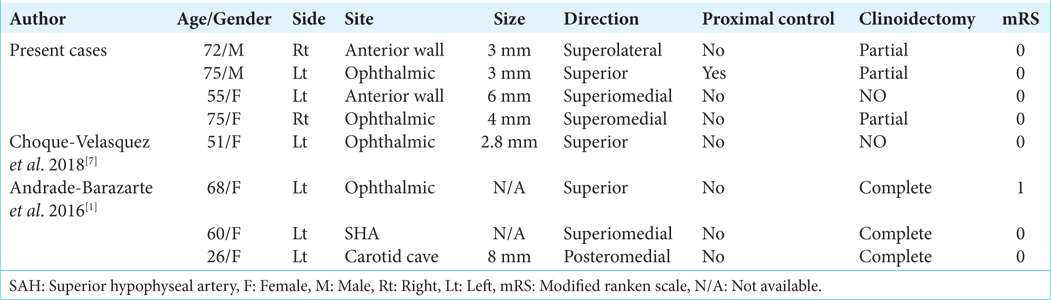
We had 49 patients with unruptured PcA aneurysms, and four of them were selected for SOK based on the preoperative simulation images. Those four unruptured PcA aneurysms have a size range (of 3~6 mm) located on the anterior wall or ophthalmic segment with dome-directed superiorly.
Three patients needed intradural anterior clinoidectomy and one patient required suction decompression through cervical ICA during the clinoidectomy.
None of the patients had residual neck, visual impairment, or neurodeficit [
Literature review
The search initially yielded 33 papers (410 aneurysms) that mentioned SOK or its variants approaches for the PcAs; then, we exclude 32 papers (406 aneurysms) due to incomplete data about aneurysm radiological characteristics, intraoperative data, or the outcome, the final review yielded two papers (four aneurysms).
The reviewed PcAs were located either in the ophthalmic segment or the subarachnoid hemorrhage (SAH) segment or in the carotid cave. Their domes were directed superior or superomedial (one was posterior), and their size was <10 mm, three of them required complete clinoidectomy only.
All of them were unruptured and there were no postoperative complications [
In total the eight patients all had unruptured aneurysms, their dome size was less than 10 mm and directed superior (one is posterior), but none is inferior. Six out of the eight cases required either complete or partial anterior clinoidectomy, the outcome for all was uneventful, and mRS 0 or 1.
DISCUSSION
In this study, we analyzed eight PcAs treated through SOK. All aneurysms were unruptured, small (<10 mm), neck located at the ophthalmic segment, SAH segments or carotid cave, and dome pointed superiorly except in one case. Six cases required anterior clinoidectomy and no one required the opening of the optic canal, one case only needs neck exposure for succession decompression, and classical microneurosurgical instruments were used, all cases were clipped completely without any neurological deficits including visual impairment. Therefore, SOK can be applied to this subset of PcAs safely.
In the past few years, endovascular therapies, mostly after the introduction of flow diversion, have gained popularity and have become a first-line treatment for such aneurysms, though associated with a lower rate of complete aneurysm occlusion and more frequent recurrences.[
Recently, SOK was introduced as an alternative approach for clipping ICA aneurysms,[
The SOK is not generally indicated to clip PcA, as suggested by Toyooka et al., who performed keyhole clipping in only two PcAs (3.7%) among the 54 PcAs, which were very small in size and required partial anterior clinoidectomy;[
The aneurysm condition whether intact or ruptured will not affect the selection of SOK practically, nevertheless, all presented eight cases were unruptured. Under the condition of brain swelling and dark operative field after subarachnoid hemorrhage, it should be difficult and dangerous to dissect ruptured PcA through a narrow SOK bone window.[
The location of the PcAs and dome direction was extremely crucial because the inferiorly or anteromedially directed aneurysm requires further manipulation of the ICA, drilling of the ACP, and optic nerve traction. This means the need for a wide area that SOK cannot provide. Most of the operated aneurysms plus the aneurysms in reviewed papers’ direction range from superomedial to lateral. No papers had discussed in detail the relationship between the dome direction and choosing the SOK.
The complete or partial anterior clinoidectomy through SOK in both presented and reviewed patients was mandatory for proximal neck security in six cases of the eight PcAs. The surgeon needs meticulous surgical technique to remove ACP through a narrow keyhole mini-craniotomy.
The residual neck (dog-ear remnant) is the most documented complication of SOK, due to a lack of visualization of the clip condition[
Proper preoperative patient selection using the radiological simulation with small aneurysmal size located in the range from lateral to the anteromedial wall or superior wall and direction superiorly or superomedial but not inferiorly considered to be favorable factors.
Limitations
The present study has several limitations. Including the patients’ data being retrospectively collected, despite being based on a prospectively maintained database, complete data were not available for 406 aneurysms clipped by SOK, and these patients were excluded from the study.
The presented cases are single-surgeon experiences that may have impacted outcomes.
In addition, formal neuro-ophthalmological evaluation was performed in all the presented cases but not all of them.
Finally, although this study demonstrates the efficacy of SOK clipping for PcAs with good clinical outcomes, further studies larger groups, and randomized control trials, are needed to establish the superiority of SOK over alternative surgical approaches for this subset of PcAs.
CONCLUSION
For unruptured PcAs, the SOK approach may be appropriate in selected patients. Unruptured, small size and superior projection are favorable characteristics for the SOK approach, which can be determined preoperatively.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Andrade-Barazarte H, Jägersberg M, Belkhair S, Tymianski R, Turel MK, Schaller K. The extended lateral supraorbital approach and extradural anterior clinoidectomy through a frontopterio-orbital window: Technical note and pilot surgical series. World Neurosurg. 2017. 100: 159-66
2. Bernat AL, Clarencon F, Andre A, Nouet A, Clémenceau S, Sourour NA. Risk factors for angiographic recurrence after treatment of unruptured intracranial aneurysms: Outcomes from a series of 178 unruptured aneurysms treated by regular coiling or surgery. J Neuroradiol. 2017. 44: 298-307
3. Caplan JM, Papadimitriou K, Yang W, Colby GP, Coon AL, Olivi A. The minipterional craniotomy for anterior circulation aneurysms: Initial experience with 72 patients. Neurosurgery. 2014. 10: 200-6 discussion 206-7
4. Chalouhi N, Jabbour P, Ibrahim I, Starke RM, Younes P, El Hage G. Surgical treatment of ruptured anterior circulation aneurysms: Comparison of pterional and supraorbital keyhole approaches. Neurosurgery. 2013. 72: 437-41 discussion 441-2
5. Chao T, Sun J, Xue H, Yu Y, Xu F. Supraorbital keyhole approach for anterior circulation aneurysms. Turk Neurosurg. 2012. 23: 4
6. Choque-Velasquez J, Hernesniemi J. Double-clip technique: An effective clipping technique for small and very small aneurysms. Surg Neurol Int. 2018. 9: 207
7. D’Urso PI, Lanzino G, Cloft HJ, Kallmes DF. Flow diversion for intracranial aneurysms: A review. Stroke. 2011. 42: 2363-8
8. Hoh BL, Carter BS, Budzik RF, Putman CM, Ogilvy CS. Results after surgical and endovascular treatment of paraclinoid aneurysms by a combined neurovascular team. Neurosurgery. 2001. 48: 78-90 discussion 89-90
9. Horiuchi T, Tanaka Y, Kusano Y, Yako T, Sasaki T, Hongo K. Relationship between the ophthalmic artery and the dural ring of the internal carotid artery. Clinical article. J Neurosurg. 2009. 111: 119-23
10. Mori K, Wada K, Otani N, Tomiyama A, Toyooka T, Tomura S. Long-term neurological and radiological results of consecutive 63 unruptured anterior communicating artery aneurysms clipped via lateral supraorbital keyhole minicraniotomy. Oper Neurosurg (Hagerstown). 2018. 14: 95-103
11. Mori K, Yamamoto T, Nakao Y, Oyama K, Esaki T, Watanabe M. Lateral supraorbital keyhole approach to clip unruptured anterior communicating artery aneurysms. Minim Invasive Neurosurg. 2008. 51: 292-7
12. Nutik S. Carotid paraclinoid aneurysms with the intradural origin and intracavernous location. J Neurosurg. 1978. 48: 526-33
13. Park J. Supraorbital keyhole approach for intracranial aneurysms: Transitioning from concerns to confidence. J Korean Neurosurg Soc. 2020. 63: 4-13
14. Rouchaud A, Leclerc O, Benayoun Y, Saleme S, Camilleri Y, D’Argento F. Visual outcomes with flow-diverter stents covering the ophthalmic artery for treatment of internal carotid artery aneurysms. AJNR Am J Neuroradiol. 2015. 36: 330-6
15. Toyooka T, Wada K, Otani N, Tomiyama A, Takeuchi S, Tomura S. Potential risks and limited indications of the supraorbital keyhole approach for clipping internal carotid artery aneurysms. World Neurosurg X. 2019. 2: 100025