- Department of Neurosurgery, St Vincent's Hospital Melbourne, Victoria, Australia
- Department of Surgery, St Vincent's Hospital, The University of Melbourne, Parkville, Victoria, Australia
- Department of Endocrinology, St Vincent's Hospital Melbourne, Victoria, Australia
- Keyhole Neurosurgery, Suite B, Level 2 Healy Wing, 41 Victoria parade, Fitzroy, VIC, Australia
Correspondence Address:
C. W. Huo
Department of Neurosurgery, St Vincent's Hospital Melbourne, Victoria, Australia
Department of Surgery, St Vincent's Hospital, The University of Melbourne, Parkville, Victoria, Australia
Keyhole Neurosurgery, Suite B, Level 2 Healy Wing, 41 Victoria parade, Fitzroy, VIC, Australia
DOI:10.4103/sni.sni_269_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: C. W. Huo, C. Caputo, Y. Y. Wang. Suprasellar keratinous cyst: A case report and review on its radiological features and treatment outcome. 22-Jan-2018;9:15
How to cite this URL: C. W. Huo, C. Caputo, Y. Y. Wang. Suprasellar keratinous cyst: A case report and review on its radiological features and treatment outcome. 22-Jan-2018;9:15. Available from: http://surgicalneurologyint.com/surgicalint-articles/suprasellar-keratinous-cyst-a-case-report-and-review-on-its-radiological-features-and-treatment-outcome/
Abstract
Background:Keratinous or epidermoid cysts (ECs) are encapsulated lesions lined by squamous cell epithelium. They comprise approximately 1% of intracranial lesions. Contrary to dermoid cysts, they lack dermal elements such as sebaceous or apocrine glands and hair follicles. The sellar region is the second most common intracranial site following the cerebellopontine angle. Here, we report a case of EC in a patient who complained of endocrine disturbances. We also performed a systematic review on previously published cases to analyze clinical and radiological characteristics and report the treatment outcomes of suprasellar ECs.
Case Description:A 42-year-old woman presented with a one-year history of amenorrhea, weight gain, severe headache, and visual disturbances for 6 months. Work-up identified an elevated prolactin level and a temporal field defect of the right eye. Magnetic resonance imaging (MRI) showed a cystic suprasellar lesion pushing on the optic chiasm. She underwent endoscopic trans-sphenoidal surgery, which confirmed a keratinous cyst on histology. Postoperatively, complete resection was confirmed on imaging. She did well although her hospital stay was prolonged due to diabetes insipidus and hypocortisolism.
Conclusion:Chronic endocrine disturbances can be the presenting complaints of a suprasellar EC, whose T1-weighted MRI appearance can be non-specific, mimicking other differential diagnoses, such as a Rathke's cleft cyst. However, the T2-weighted MRI appearances of ECs are generally hyper-intense and lesions show diffusion restriction. Treatment is surgical and yields good outcomes in most cases reported.
Keywords: Endoscopic, epidermoid cyst, keratinous cyst, skull base, suprasellar
INTRODUCTION
The suprasellar cistern is a cerebrospinal fluid (CSF)-filled space below the third ventricle with a floor formed by the dura of the diaphragma sellae. Normally, this space contains the optic nerves and chiasm, the tuber cinereum and floor of the anterior third ventricle, the hypothalamus, and the vessels forming the circle of Willis.[
Epidermoid cysts (ECs) are also known as keratinous cysts; when they occur within the CNS, they had been erroneously referred as neurenteric cysts[
Here, we report a suprasellar EC case in a patient presenting with endocrine and visual compromise. Histological examination of the excised tumor confirmed a keratinous cyst. To put this observation into appropriate context, we systematically reviewed all previously published English literature on ECs of the sellar region to assess the radiological features of such lesions and their treatment outcomes.
CASE DESCRIPTION
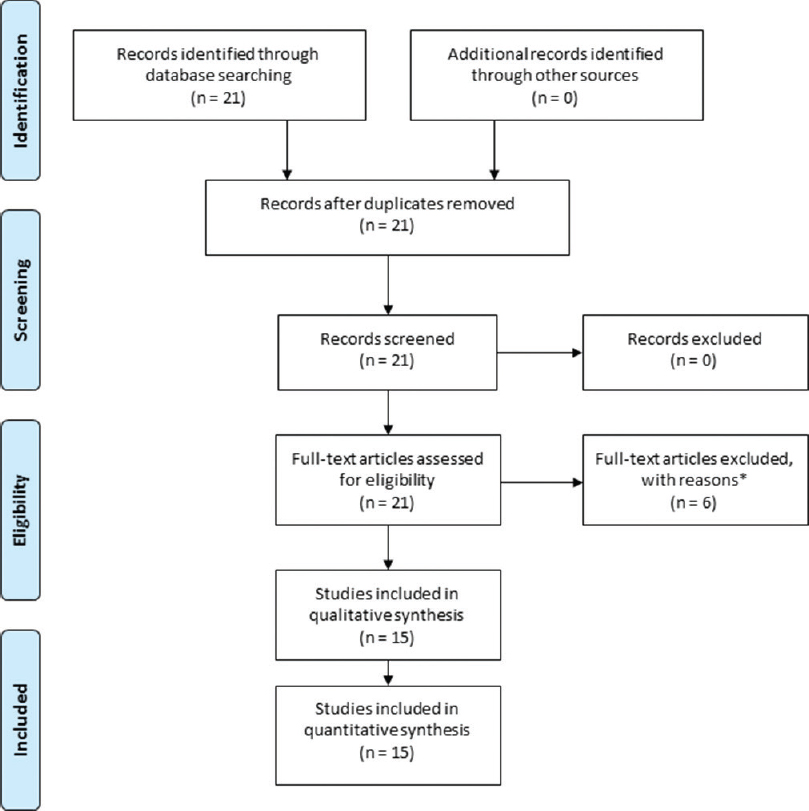
The keywords “epidermoid cyst” OR “keratinous cyst” AND “*sellar” were used to perform an electronic search of the English literature indexed in PubMed and OVID-Medline databases on human studies (adults and children) without any limited time frame. Collection of case reports and series followed the PRISMA guideline[
Regarding the case report, a signed written consent was obtained from the patient for the publication of her case. Information on the presenting history, radiological appearance, surgical management, and pathological reports of her suprasellar lesion were collected from the medical records and directly obtained from the patient.
The patient was a 42-year-old administrative officer who initially presented with a one-year history of amenorrhoea to her family physician. Blood tests revealed normal hormonal functions other than an elevated prolactin level at 875 mIU/L (normal range: 110–560 mIU/L). The mildly increased secretion of prolactin is most likely caused by a disinhibition of lactotropes from impaired dopamine delivery to the pituitary, as a result of the tumor compressing on the pituitary stalk – the Stalk effect; whereas in cases of prolactinomas, the value of prolactin usually exceeds 1,000 mIU/L.[
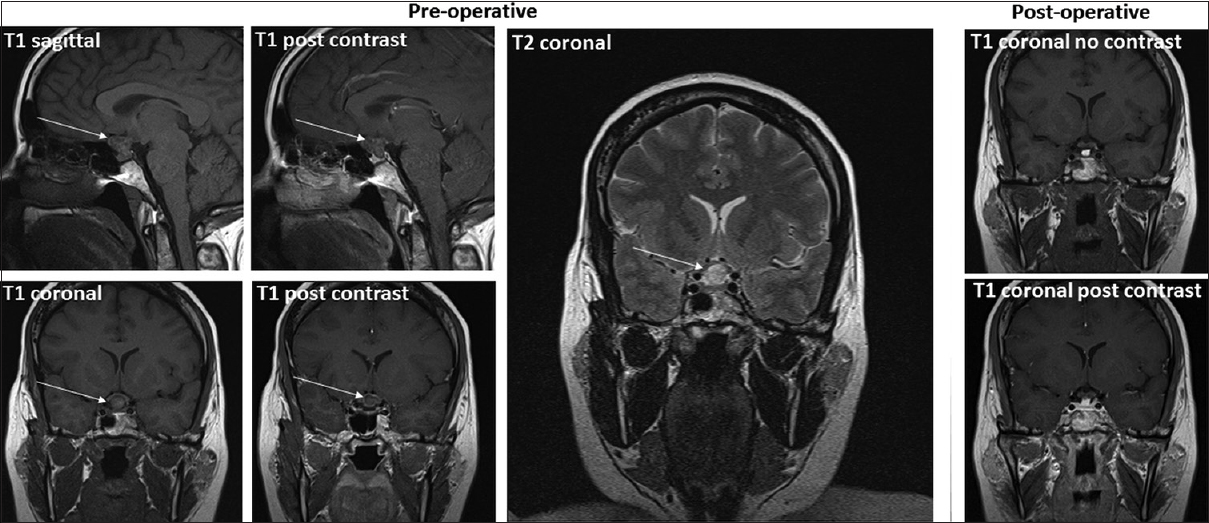
Given her symptoms, the patient was scheduled for endoscopic trans-sphenoidal surgery. A repeat MRI prior to surgery showed a T1 hypo-intense, T2 hyper-intense, peripherally enhancing lesion within the sella extending into the suprasellar space and displacing the optic chiasm. Ill-defined intrinsic T1 hyper-intense signal was seen within the lesion [
Figure 2
Pre- and postoperative MRI images of the suprasellar lesion. Preoperative images show a T1 hypo-intense and T2 hyper-intense suprasellar lesion with periphery enhancement post magnevist contrast administration. Postoperative T1-weighted images demonstrate the fat graft but no residual tumor. White arrows indicate the lesion
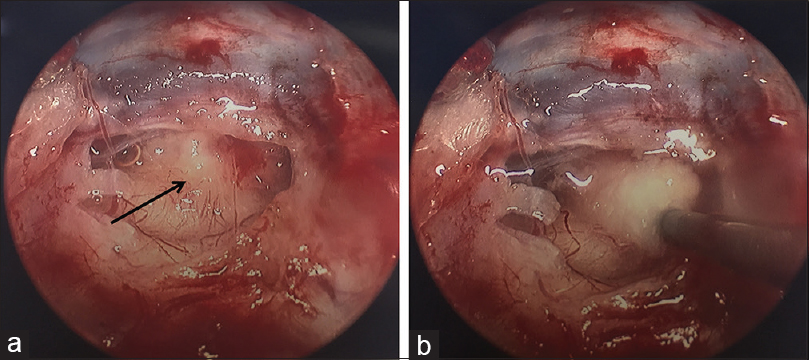
Figure 3
Intraoperative images of the suprasellar lesion (a) Endoscopic pterional view of the laminar terminalis. ICA: internal carotid artery, MCA: middle cerebral artery, R: right, ACA: anterior cerebral artery. (b) A zoomed-in view of A. L: left, ACOM: anterior communicating artery, RAH: recurrent artery of Heubner
DISCUSSION
Clinical presentation
ECs are congenital, indolent tumors that may remain asymptomatic for years prior to detection.[
Typically, ECs present in the fourth to fifth decades of life,[
Radiographic characteristics
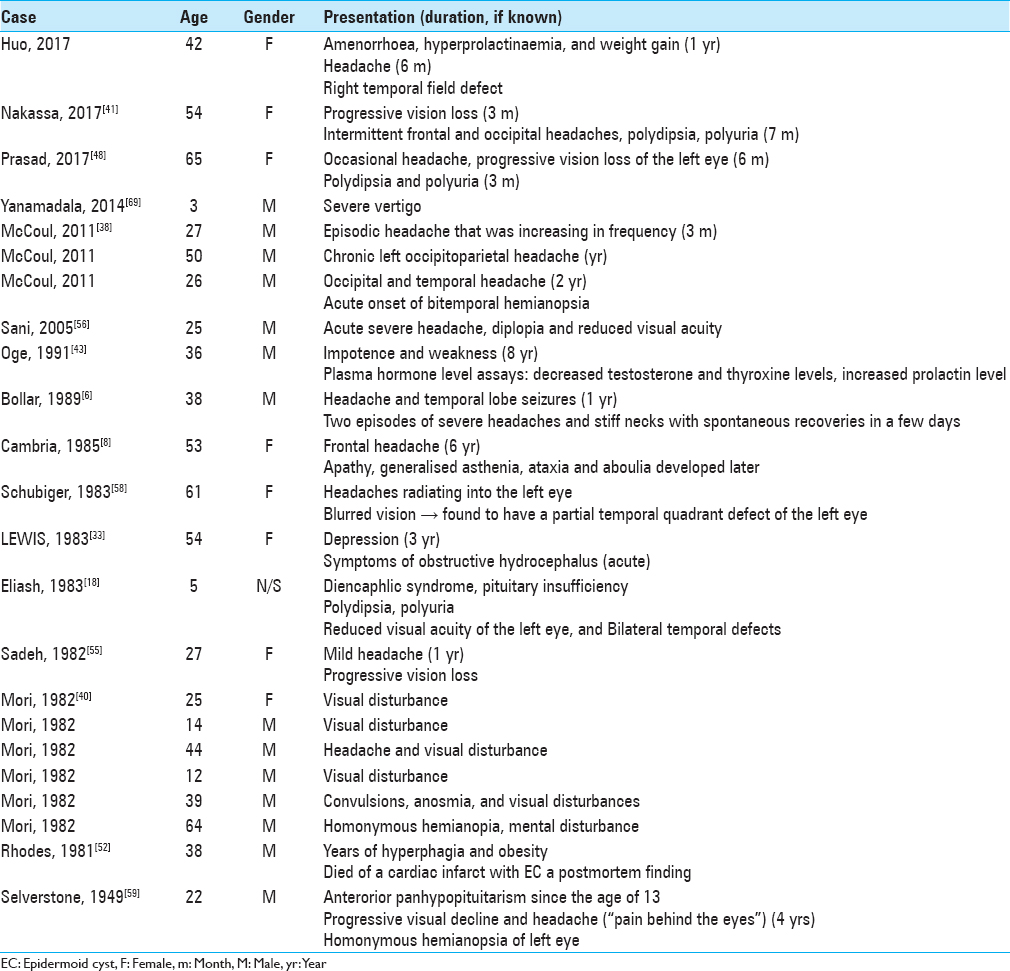
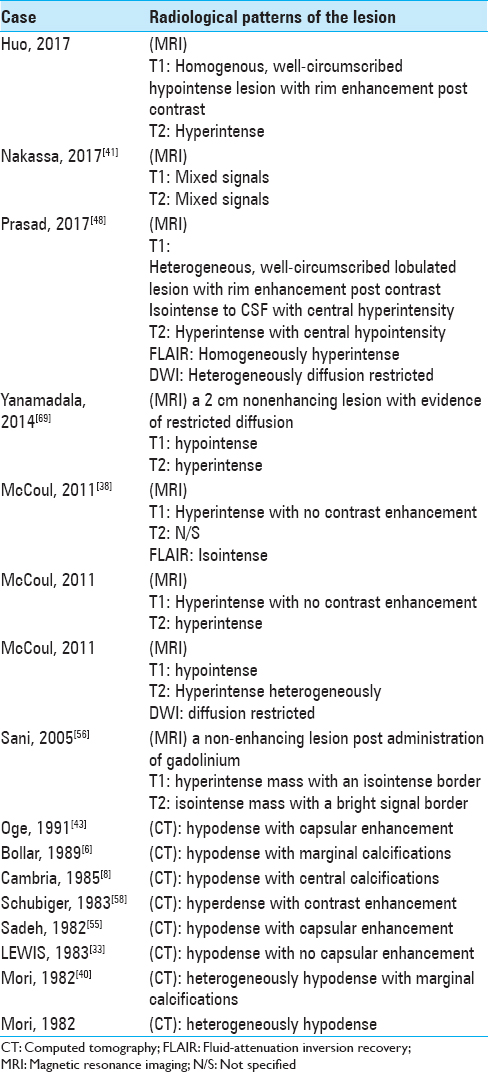
Reported imaging characteristics of ECs are summarized in
On CT, ECs most often appear hypo-dense due to the low absorptive value of their fat content[
In comparison to dermoid cysts, the latter typically appear hyper-intense on T1, with ECs showing hyper-intense signal on T2;[
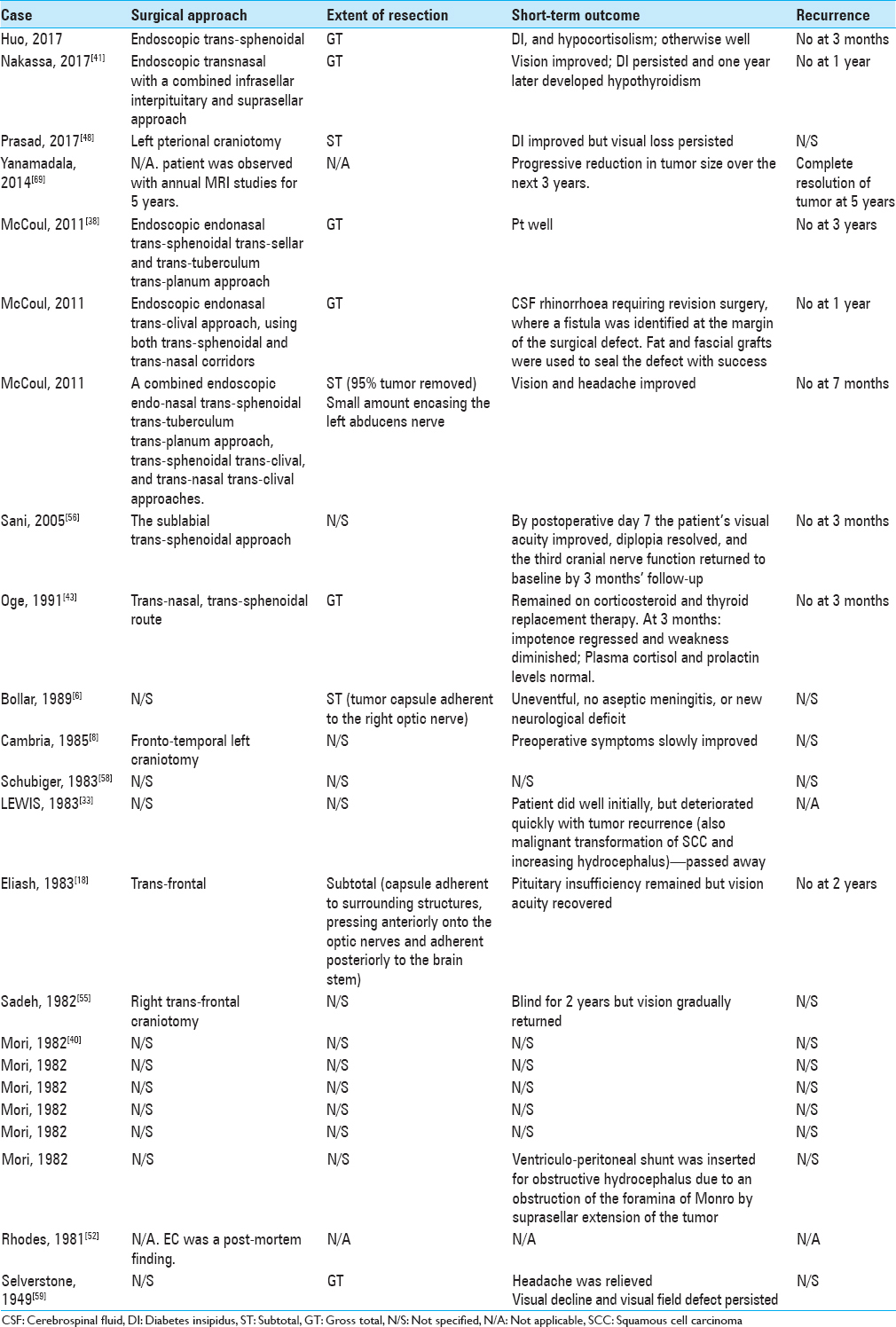
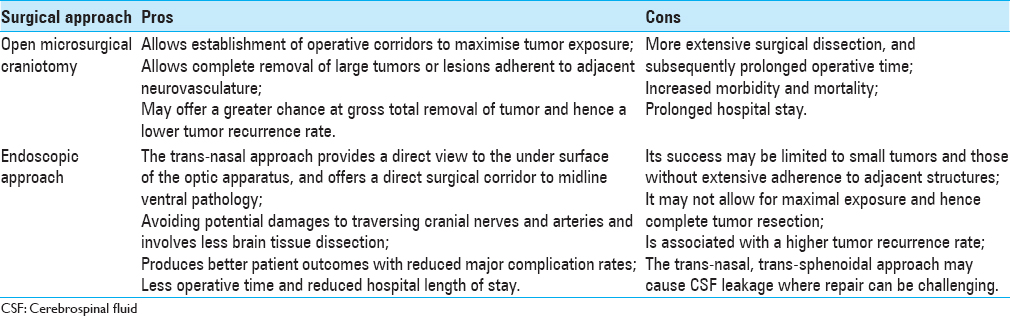
Surgical options and outcomes
Complete excision of the lesion and its capsule is important for satisfactory long-term results while preserving function and quality-of-life of the patient.[
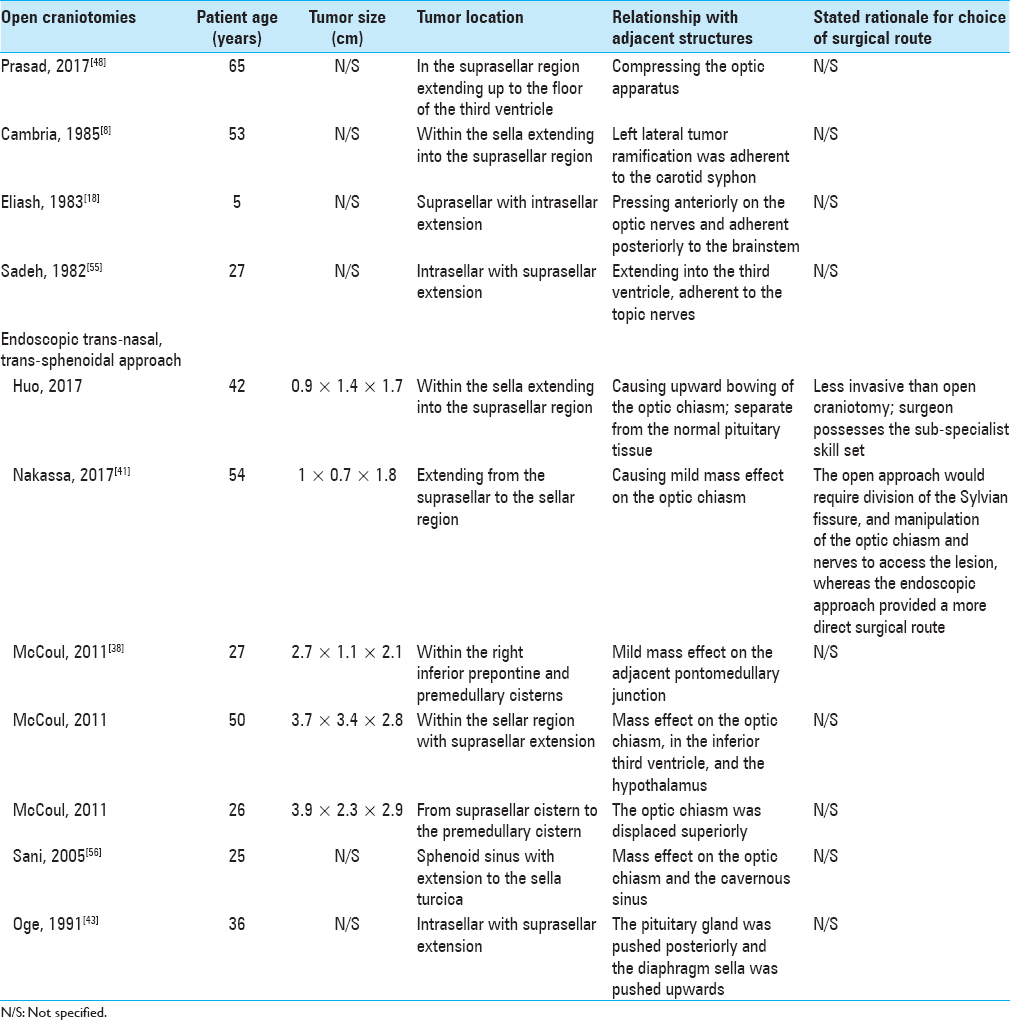
As summarized in
Despite colloid cyst of the anterior third ventricle being a different cystic tumor in a slightly distinct anatomical location compared with EC, due to its more common incidence,[
Overall, postoperative recovery after EC removal was satisfactory, although persistent DI and other endocrine disturbances had been reported [
Histogenesis
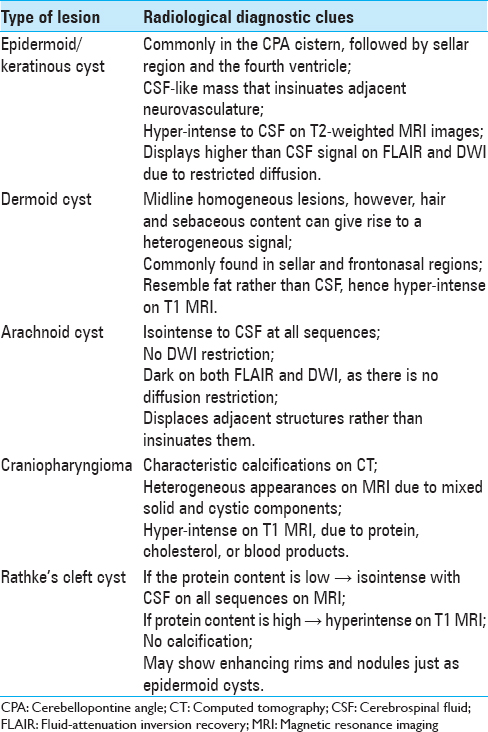
While craniopharyngiomas contain cholesterol-rich fluids, Rathke's cleft cysts are developmental remnants of the formation of Rathke's cleft and contain mucoid colloid.[
Lepoire and Pertuiset categorized ECs into three groups, retrosellar, suprasellar, and intraventricular based on the embryonic relation to the carotid, basilar, and choroid arteries.[
CONCLUSION
Epidermoid or keratinous cysts are rare CNS lesions that typically present clinically in middle-aged adults. Four cases have also been reported in children. Although these ECs are slow-growing and benign lesions, various neurological symptoms can occur based on location and include endocrinopathies, cranial nerve palsies, and obstructive hydrocephalus. For ECs in the suprasellar region, we report a case of a patient who presented with one-year history of endocrine disturbances prior to clinical diagnosis. This differs from most cases in the existing literature, where endocrine symptoms are less common compared to headaches and visual disturbances.
After conducting a focused systematic review on suprasellar ECs, pooled analysis showed that the distinct radiographic feature of a suprasellar EC is its diffusion restriction on diffusion-weighted imaging in combination with fluid-attenuated inversion recovery characteristics and hyper-intensity on T2-weighted MRI.
GTR of the lesion including its capsule is the optimal treatment to alleviate symptoms and to prevent recurrence. Endoscopic trans-nasal and trans-sphenoidal approaches have become the mainstream surgical route and are suitable for most cases. Attention needs to be paid to the possibilities of postoperative DI, various pituitary deficiencies, and CSF leakage.
Informed consent
Informed consent was obtained from the individual participant included in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Achard JM, Lallement PY, Veyssier P. Recurrent aseptic meningitis secondary to intracranial epidermoid cyst and Mollaret's meningitis: Two distinct entities or a single disease? A case report and a nosologic discussion. Am J Med. 1990. 89: 807-10
2. Agarwal S, Rishi A, Suri V, Sharma MC, Satyarthi GD, Garg A. Primary intracranial squamous cell carcinoma arising in an epidermoid cyst--a case report and review of literature. Clin Neurol Neurosurg. 2007. 109: 888-91
3. Baumann CH, Bucy PC. Paratrigeminal epidermoid tumors. J Neurosurg. 1956. 13: 455-68
4. Bergsneider M, Mirsadraei L, Yong WH, Salamon N, Linetsky M, Wang MB. The pituitary stalk effect: Is it a passing phenomenon?. J Neurooncol. 2014. 117: 477-84
5. Bergui M, Zhong J, Bradac GB, Sales S. Diffusion-weighted images of intracranial cyst-like lesions. Neuroradiology. 2001. 43: 824-9
6. Bollar A, Gelabert M, Allut AG, Prieto A. Spontaneous rupture of epidermoid cyst. Neurochirurgia (Stuttg). 1989. 32: 123-4
7. Bostroem E. Ueber die pialen Epidermoide, Dermoide, und Lipome und duralen Dermoide. Zentralbl Allg Pathol. 1897. 8: 1-98
8. Cambria S, Cardia E, Manti G, Galatioto S. Optical-chiasmatic region epidermoid with a suprasellar and prepontina region cysticercosis. J Neurosurg Sci. 1985. 29: 51-6
9. Cavazzani P, Ruelle A, Michelozzi G, Andrioli G. Spinal dermoid cysts originating intracranial fat drops causing obstructive hydrocephalus: Case reports. Surg Neurol. 1995. 43: 466-9
10. Chakraborty S, Priamo F, Loven T, Li J, Insinga S, Schulder M. Supratentorial Neurenteric Cysts: Case Series and Review of Pathology, Imaging, and Clinical Management. World Neurosurg. 2016. 85: 143-52
11. Chen CT, Lai HY, Jung SM, Lee CY, Wu CT, Lee ST. Neurenteric Cyst or Neuroendodermal Cyst? Immunohistochemical Study and Pathogenesis. World Neurosurg. 2016. 96: 85-90
12. Chen CY, Wong JS, Hsieh SC, Chu JS, Chan WP. Intracranial epidermoid cyst with hemorrhage: MR imaging findings. AJNR Am J Neuroradiol. 2006. 27: 427-9
13. Chowdhury FH, Haque MR, Sarker MH. Intracranial epidermoid tumor; microneurosurgical management: An experience of 23 cases. Asian J Neurosurg. 2013. 8: 21-8
14. Cohen JE, Abdallah JA, Garrote M. Massive rupture of suprasellar dermoid cyst into ventricles. Case illustration. J Neurosurg. 1997. 87: 963-
15. Connor SE, Penney CC. MRI in the differential diagnosis of a sellar mass. Clin Radiol. 2003. 58: 20-31
16. Critchley M, Ferguson FR. The cerebrospinal epidermoids (cholesteatomata). Brain. 1928. 51: 334-84
17. Cruveilhier J.editorsAnatomie Pathologique de Corps Humain. Paris: JB Bailliere; 1829. p. 341-
18. Eliash A, Roitman A, Karp M, Reichental E, Manor RS, Shalit M. Diencephalic syndrome due to a suprasellar epidermoid cyst. Child's Brain. 1983. 10: 414-8
19. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med. 2013. 2: 38-43
20. Gelabert-Gonzalez M. [Intracranial epidermoid and dermoid cysts]. Rev Neurol. 1998. 27: 777-82
21. Glasauer FE, Levy LF, Auchterlonie WC. Congenital inclusion dermoid cyst of the anterior fontanel. J Neurosurg. 1978. 48: 274-8
22. Gormley WB, Tomecek FJ, Qureshi N, Malik GM. Craniocerebral epidermoid and dermoid tumours: A review of 32 cases. Acta Neurochir (Wien). 1994. 128: 115-21
23. Grondin RT, Hader W, MacRae ME, Hamilton MG. Endoscopic versus microsurgical resection of third ventricle colloid cysts. Can J Neurol Sci. 2007. 34: 197-207
24. Gualdi GF, Di Biasi C, Trasimeni G, Pingi A, Vignati A, Maira G. Unusual MR and CT appearance of an epidermoid tumor. AJNR Am J Neuroradiol. 1991. 12: 771-2
25. Hakyemez B, Aksoy U, Yildiz H, Ergin N. Intracranial epidermoid cysts: Diffusion-weighted, FLAIR and conventional MR findings. Eur J Radiol. 2005. 54: 214-20
26. Hamer J. Diagnosis by computerized tomography of intradural dermoid with spontaneous rupture of the cyst. Acta Neurochir (Wien). 1980. 51: 219-26
27. Hamlat A, Hua ZF, Saikali S, Laurent JF, Gedouin D, Ben-Hassel M. Malignant transformation of intra-cranial epithelial cysts: Systematic article review. J Neurooncol. 2005. 74: 187-94
28. Hori T, Kawamata T, Amano K, Aihara Y, Ono M, Miki N. Anterior interhemispheric approach for 100 tumors in and around the anterior third ventricle. Neurosurgery. 2010. 66: 65-74
29. Inoue Y, Ohata K, Nakayama K, Haba T, Shakudo M. An unusual middle fossa interdural epidermoid tumor. Case report. J Neurosurg. 2001. 95: 902-4
30. Kallmes DF, Provenzale JM, Cloft HJ, McClendon RE. Typical and atypical MR imaging features of intracranial epidermoid tumors. AJR Am J Roentgenol. 1997. 169: 883-7
31. Kato K, Ujiie H, Higa T, Hayashi M, Kubo O, Okada Y. Clinical presentation of intracranial epidermoids: A surgical series of 20 initial and four recurred cases. Asian J Neurosurg. 2010. 5: 32-40
32. Lepoire J, Pertuiset B. [Cranioencephalic epidermoid cysts]. Neurochirurgie. 1957. 3: 319-22
33. Lewis AJ, Cooper PW, Kassel EE, Schwartz ML. Squamous cell carcinoma arising in a suprasellar epidermoid cyst. Case report. J Neurosurg. 1983. 59: 538-41
34. Li F, Zhu S, Liu Y, Chen G, Chi L, Qu F. Hyperdense intracranial epidermoid cysts: A study of 15 cases. Acta Neurochir (Wien). 2007. 149: 31-9
35. Lynch JC, Aversa A, Pereira C, Nogueira J, Goncalves M, Lopes H. Surgical strategy for intracranial dermoid and epidermoid tumors: An experience with 33 Patients. Surg Neurol Int. 2014. 5: 163-
36. Manno NJ, Uihlein A, Kernohan JW. Intraspinal epidermoids. J Neurosurg. 1962. 19: 754-65
37. Margetis K, Souweidane MM. Endoscopic treatment of intraventricular cystic tumors. World Neurosurg. 2013. 79: S19 e11-11
38. McCoul ED, Chow S, Lee DL, Anand VK, Schwartz TH. Endoscopic endonasal approach for resection of ventral skull base keratinaceous cysts. Int Forum Allergy Rhinol. 2012. 2: 258-63
39. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010. 8: 336-41
40. Mori K, Handa H, Moritake K, Takeuchi J, Nakano Y. Suprasellar epidermoid. Neurochirurgia (Stuttg). 1982. 25: 138-42
41. Nakassa AC, Chabot JD, Snyderman CH, Wang EW, Gardner PA, Fernandez-Miranda JC. Complete endoscopic resection of a pituitary stalk epidermoid cyst using a combined infrasellar interpituitary and suprasellar endonasal approach: Case report. J Neurosurg. 2017. p. 1-7
42. Ochi M, Hayashi K, Hayashi T, Morikawa M, Ogino A, Hashmi R. Unusual CT and MR appearance of an epidermoid tumor of the cerebellopontine angle. AJNR Am J Neuroradiol. 1998. 19: 1113-5
43. Oge K, Ozgen T. Transsphenoidal removal of an intra- and suprasellar epidermoid cyst. Neurochirurgia (Stuttg). 1991. 34: 94-6
44. Osborn AG, Preece MT. Intracranial cysts: Radiologic-pathologic correlation and imaging approach. Radiology. 2006. 239: 650-64
45. Patibandla MR, Yerramneni VK, Mudumba VS, Manisha N, Addagada GC. Brainstem epidermoid cyst: An update. Asian J Neurosurg. 2016. 11: 194-200
46. Pikis S, Margolin E. Malignant transformation of a residual cerebellopontine angle epidermoid cyst. J Clin Neurosci. 2016. 33: 59-62
47. Pisanesehi M, Kapoor G. Imaging of sella and parasellar region. Neuroimaging Clin N Am. 2005. 15: 203-19
48. Prasad GL, Pavithra P. Suprasellar Epidermoid Cyst with Atypical Imaging Findings. World Neurosurg. 2017. 98: 870 e871-870 e873
49. Rao VJ, James RA, Mitra D. Imaging characteristics of common suprasellar lesions with emphasis on MRI findings. Clin Radiol. 2008. 63: 939-47
50. Reddy A, Kreicher KL, Patel NA, Schantz S, Shinhar S. Pediatric epidermoid cysts masquerading as ranulas: A case series. Int J Pediatr Otorhinolaryngol. 2016. 81: 26-8
51. Ren X, Lin S, Wang Z, Luo L, Jiang Z, Sui D. Clinical, radiological, and pathological features of 24 atypical intracranial epidermoid cysts. J Neurosurg. 2012. 116: 611-21
52. Rhodes RH, Davis RL, Beamer YB, Marantz C. A suprasellar epidermoid cyst with symptoms of hypothalamic involvement: Case report and a review of pathogenetic mechanisms. Bull Los Angeles Neurol Soc. 1981. 46: 26-32
53. Ruscalleda J. Imaging of parasellar lesions. Eur Radiol. 2005. 15: 549-59
54. Rutherford SA, Leach PA, King AT. Early recurrence of an intracranial epidermoid cyst due to low-grade infection: Case report. Skull Base. 2006. 16: 109-16
55. Sadeh M, Goldhammer Y, Shacked I, Tadmor R, Godel V. Basal encephalocele associated with suprasellar epidermoid cyst. Arch Neurol. 1982. 39: 250-2
56. Sani S, Smith A, Leppla DC, Ilangovan S, Glick R. Epidermoid cyst of the sphenoid sinus with extension into the sella turcica presenting as pituitary apoplexy: Case report. Surg Neurol. 2005. 63: 394-7
57. Schiefer TK, Link MJ. Epidermoids of the cerebellopontine angle: A 20-year experience. Surg Neurol. 2008. 70: 584-90
58. Schubiger O, Valavanis A, Gessaga E. Dense suprasellar epidermoid cyst. A case report. Neuroradiology. 1983. 24: 269-71
59. Selverstone B, Kubik CS. Suprasellar epidermal cyst. N Engl J Med. 1949. 241: 309-11
60. Skinner DC. Rethinking the stalk effect: A new hypothesis explaining suprasellar tumor-induced hyperprolactinemia. Med Hypotheses. 2009. 72: 309-10
61. Smith JK. Parasellar tumors: Suprasellar and cavernous sinuses. Top Magn Reson Imaging. 2005. 16: 307-15
62. Tan LA, Kasliwal MK, Harbhajanka A, Kellogg RG, Arvanitis LD, Munoz LF. Hyperdense suprasellar mass: An unusual radiological presentation of intracranial dermoid cyst. J Clin Neurosci. 2015. 22: 1208-10
63. Timmer FA, Sluzewski M, Treskes M, van Rooij WJ, Teepen JL, Wijnalda D. Chemical analysis of an epidermoid cyst with unusual CT and MR characteristics. AJNR Am J Neuroradiol. 1998. 19: 1111-2
64. Toglia JU, Netsky MG, Alexander E. Epithelial (epidermoid) tumors of the cranium. Their common nature and pathogenesis. J Neurosurg. 1965. 23: 384-93
65. Tsuruda JS, Chew WM, Moseley ME, Norman D.editors. Diffusion-weighted MR imaging of the brain: Value of differentiating between extraaxial cysts and epidermoid tumors. AJNR Am J Neuroradiol. 1990. 11: 925-31
66. Ulivieri S, Oliveri G, Filosomi G, Miracco C. Intracranial epidermoid cyst: Case report. Ann Ital Chir. 2008. 79: 445-6
67. Yadav YR, Yadav N, Parihar V, Kher Y, Ratre S. Management of colloid cyst of third ventricle. Turk Neurosurg. 2015. 25: 362-71
68. Yamakawa K, Shitara N, Genka S, Manaka S, Takakura K. Clinical course and surgical prognosis of 33 cases of intracranial epidermoid tumors. Neurosurgery. 1989. 24: 568-73
69. Yanamadala V, Lin N, Walcott BP, Baird LC, Smith ER. Spontaneous regression of an epidermoid cyst of the cavernous sinus. J Clin Neurosci. 2014. 21: 1433-5
70. Yasargil MG, Abernathey CD, Sarioglu AC. Microneurosurgical treatment of intracranial dermoid and epidermoid tumors. Neurosurgery. 1989. 24: 561-7
71. Yilmazlar S, Kocaeli H, Cordan T. Brain stem stroke associated with epidermoid tumours: Report of two cases. J Neurol Neurosurg Psychiatry. 2004. 75: 1340-2