- Department of Neurosurgery, Osaka Police Hospital, Tennoji, Osaka, Japan.
Correspondence Address:
Daisuke Wajima
Department of Neurosurgery, Osaka Police Hospital, Tennoji, Osaka, Japan.
DOI:10.25259/SNI_193_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daisuke Wajima, Shuta Aketa, Taiji Yonezawa. Supratentorial convexity schwannoma unrelated to cranial nerves: Case report and review of the literature. 06-Jun-2020;11:143
How to cite this URL: Daisuke Wajima, Shuta Aketa, Taiji Yonezawa. Supratentorial convexity schwannoma unrelated to cranial nerves: Case report and review of the literature. 06-Jun-2020;11:143. Available from: https://surgicalneurologyint.com/surgicalint-articles/10071/
Abstract
Background: Intracranial schwannoma not related to cranial nerves is rare entity, and difficult to be diagnosed preoperatively. Here, we experienced a case of convexity schwannoma mimicking convexity meningioma, and discuss about the characteristics of such cases based on the past published reports.
Case Description: A 48-year-old man presented with a sudden onset of seizures. Brain magnetic resonance image (MRI) revealed a small mass lesion in the peripheral aspect of the right parieto-frontal lobe. The mass was isointense on T1-weighted and hyperintense on T2-weighted MRI, with homogenous enhancement after contrast medium administration. After the feeder embolization on the previous day, removal of the tumor was performed. The tumor revealed a well-demarcated, firm, spherical tumor beyond, and beneath the dura and was relatively easy to be separated from the brain. Histologically, the tumor was observed to be in subarachnoid space extending to outer space of dura-mater, intimately attached to the pia mater. The histological diagnosis was schwannoma.
Conclusion: In our case, MRI findings are similar to convexity meningioma; however, the pathological diagnosis was schwannoma. Cerebral convexity is an extremely rare location for schwannoma. We emphasize that schwannoma, not related to cranial nerves, may arise in the subdural convexity space.
Keywords: Convexity schwannoma, Unrelated cranial nerve origin, Review
INTRODUCTION
The majority of intracranial schwannomas arises from the cranial nerves and usually occurs in the middle decades of life. Intracranial schwannoma not associated with cranial nerves is infrequent and usually involves in neurofibromatosis, and occurs among children or young adults.[
CASE REPORT
A 48-year-old man experienced a sudden onset of seizures in his left lower limb. He had never previously experienced such an episode. Gadolinium-enhanced MRI (Gd-MRI) revealed a small, homogenous-enhancing mass in the peripheral aspect of the right parieto-frontal lobe. The patient was referred to our hospital for further evaluation. The results of the physical examination performed at the time of admission were unremarkable. No cutaneous stigmata of neurofibromatosis Type I was observed. A neurological examination revealed no significant findings.
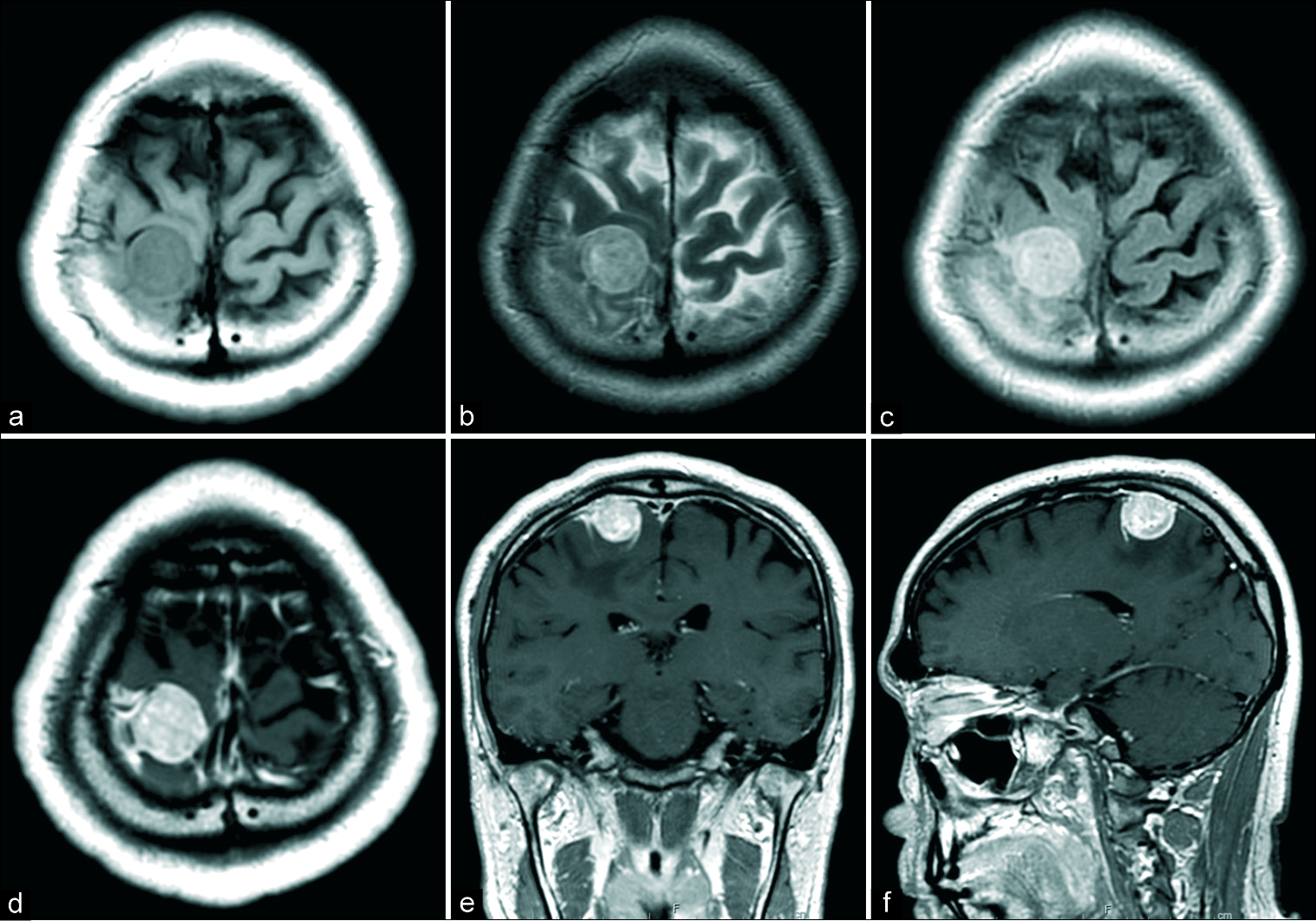
The lesion appeared as a well-defined, isosignal intensity area on T1-weighted MRI [
Figure 1:
Preoperative magnetic resonance images (MRI). Preoperative MRI showed right frontal mass lesion. The lesion was isointense in T1-weighted MRI (a), hyperintense in T2-weighted MRI (b), hyperintense in fluid attenuation inversion recovery image (c), and homogenous enhanced with gadolinium intravenous injection (d: Axial image, e: Coronal image, and f: Sagittal image).
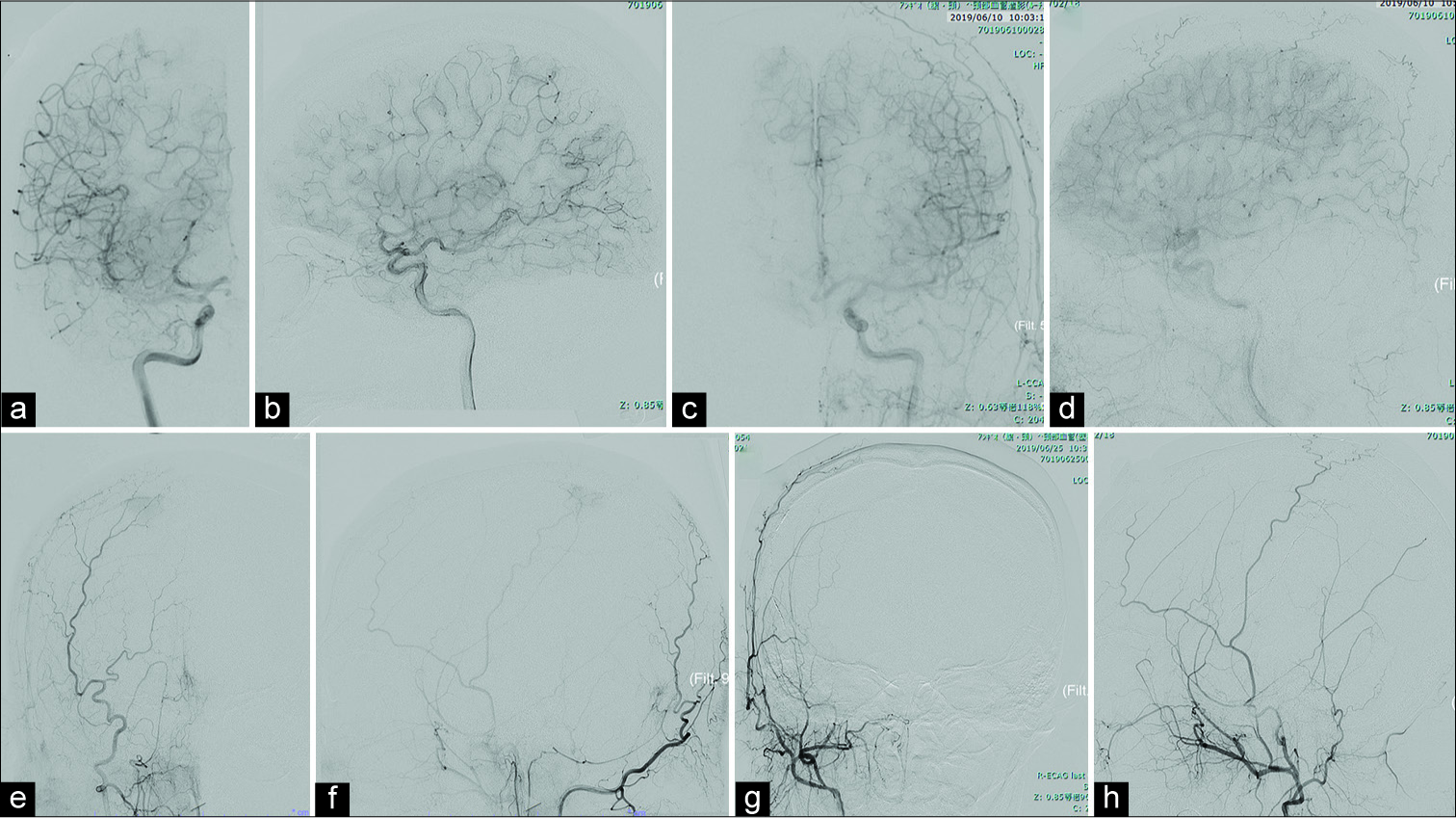
Figure 2:
Digital subtraction findings (DSA). Preoperative DSA showed some feeding arteries (right middle cerebral artery branch [Right carotid artery angiography (Rt CAG)], a: Axial view, b: Lateral view), right anterior cerebral artery branch (Lt CAG, c: Axial view, d: Lateral view), and right middle meningeal artery (MMA) branch (Rt external CAG, e: Axial view, f: lateral view). Preoperative feeder embolization targeted to right MMA branch was performed (Rt external CAG, g: Axial view, h: Lateral view).
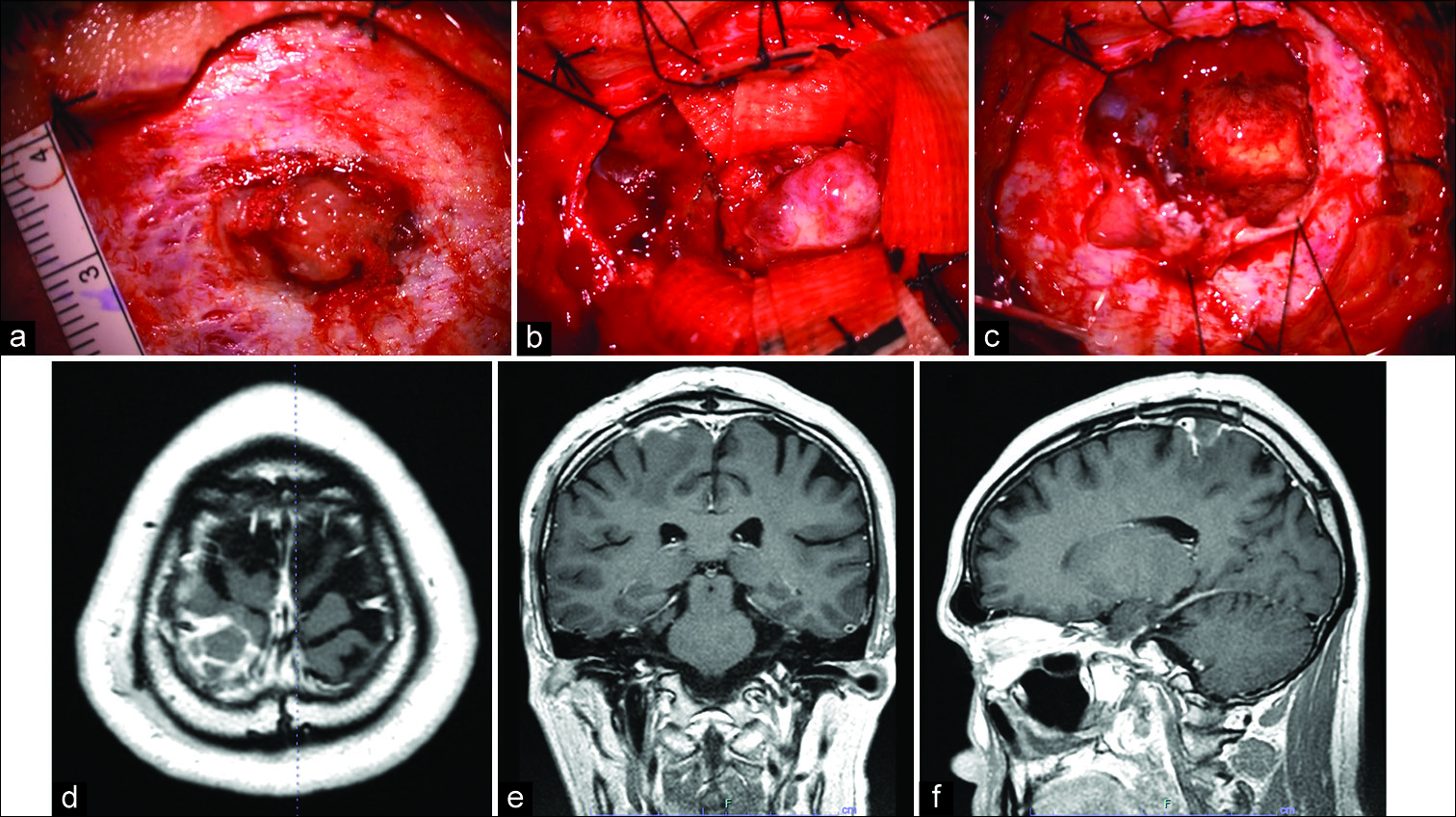
Our preoperative diagnosis was an extra-axial tumor, such as convexity meningioma or other tumors. A whole-body evaluation revealed no evidence of malignancy. The patient underwent a parietal craniotomy, with total resection of the tumor. During surgery, tumoral invasion beyond the dura mater was observed [
Figure 3:
Intraoperative findings and postoperative MRI findings. During surgery, tumoral invasion beyond the dura mater was observed (a). When the dura was opened, a well-demarcated, firm, spherical tumor was observed beneath the arachnoid membrane. The tumor was elastic, hard, grayish in color, and loosely adherent to but separable from the dura mater (b). The tumor was totally removed piece by piece, with the adjacent brain tissue and dura (c). Postoperative MRI showed the total removal of the tumor (d: Axial view, e: Coronal view, f: sagittal view).
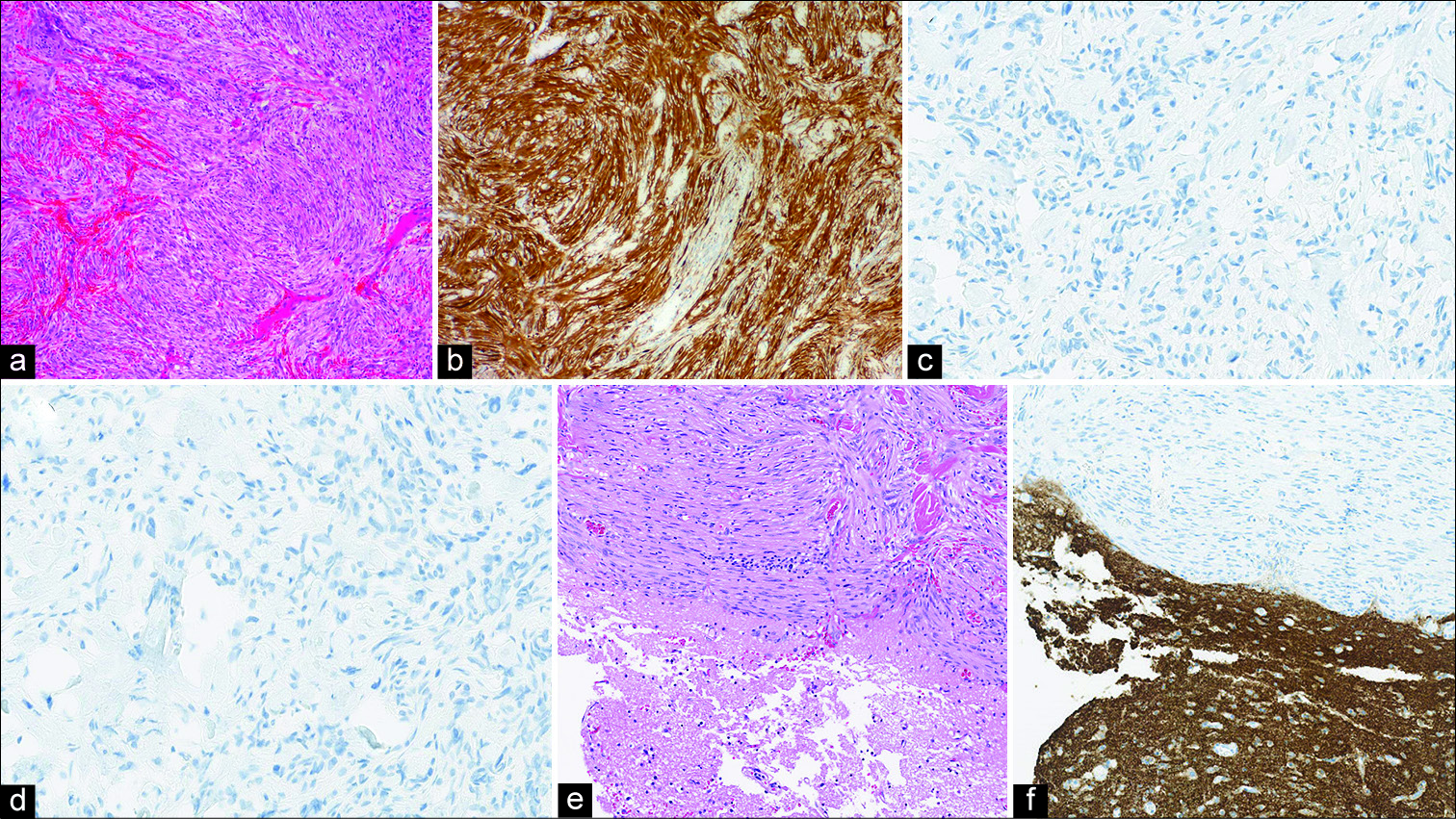
In light-microscopic assessments, the tumor was composed predominantly of Antoni Type A, and intermingled with Antoni Type B [
Figure 4:
Histology findings. Compact bundles of thin, elongated, finely fibrillated cells, which tend to exhibit nuclear palisading (Antoni Type A) and which are intermingled with a looser pattern of Antoni Type B, can be observed. The adjacent cortex contained no tumor cells (a: hematoxylin and eosin (HE); original magnification, ×10). Immunohistochemical staining was positive for S-100 protein (b) but negative for glial fibrillary acidic protein (GFAP), cluster of differentiation (CD) 34, epithelial membrane antigen (EMA) (c), signal transducer and activator of transcription 6 (STAT 6) (d), progesterone receptor (PgR), and synaptophysin. The histopathological diagnosis was schwannoma. The tumor was observed to be in the subarachnoid space extending to the outer space of dura-mater, intimately attached to the pia mater (e: HE staining, f: Synaptophysin immunostaining).
DISCUSSION
Intracranial schwannoma are benign tumors that typically arise from the Schwann cells en-sheathing the axons beginning at the point of their exit from the pia mater. They usually develop among middle-age adults. Intracranial schwannomas comprise 6 to 8% of intracranial tumors.[
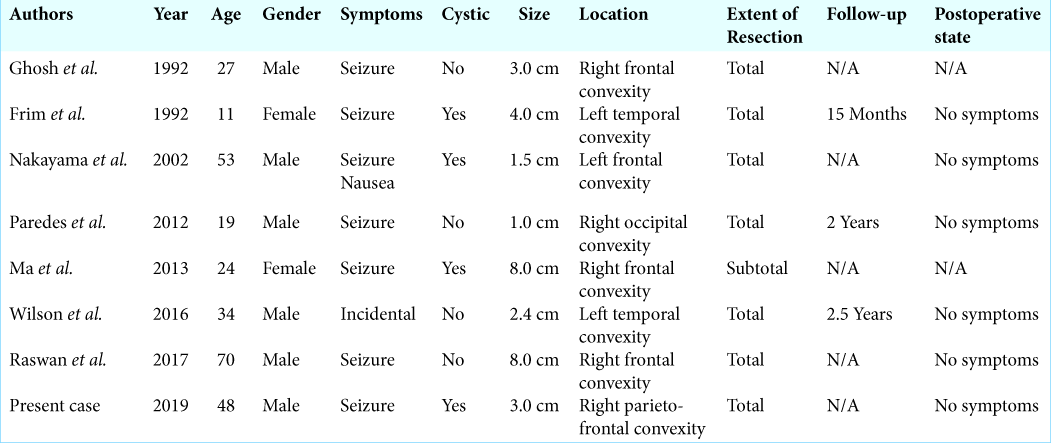
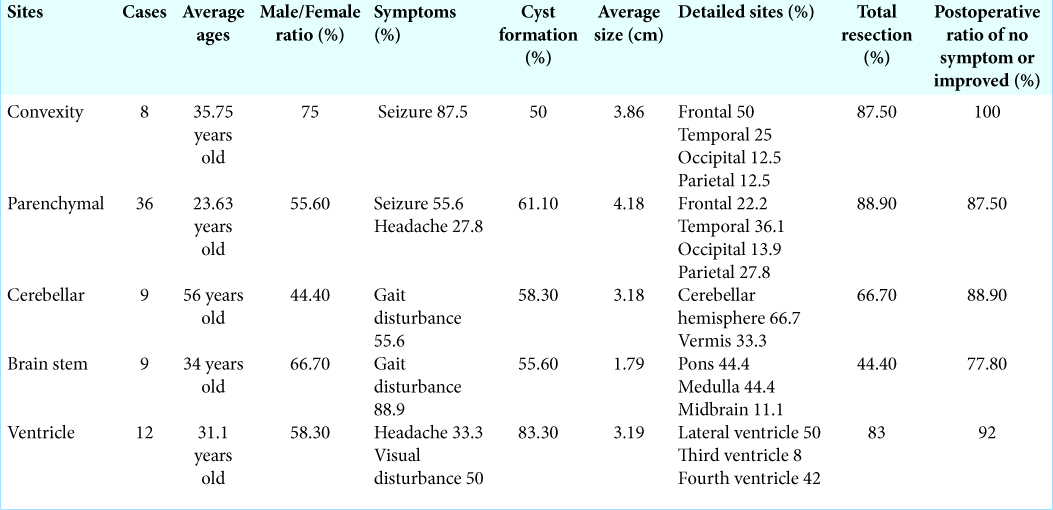
Intracranial schwannomas not related to cranial nerves are relatively rare; 74 cases have been reported.[
It is reasonable to consider the possibility that intracranial schwannomas not related to cranial nerves may develop from ectopic Schwann cells, because there are no Schwann cells in the central nervous system. Proposed hypotheses include: (1) the presence of Schwann cells in the autonomic nerves of the subarachnoid vessels, (2) the conversion of pial cells into Schwann cells, and (3) the presence of sensory nerves in the meninges of the convexity.
Although several hypotheses have been proposed to explain the origin of ectopic Schwann cells within the central nervous system, the histological origin of these tumors remains controversial. Proposed hypotheses include:
Schwann cell hyperplasia
Riggs and Clary proposed Schwann cell hyperplasia as the origin of intracranial neurinomas;[
Schwannosis
The term “Schwannosis” was introduced by Russell and Rubinstein,[
Reactive changes after injury
Nelson and Rennels[
The possible differentiation of multipotent mesenchymal cell into Schwann cells (Neural crest origin)
Feigin and Ogata[
In our case, the tumor was present not within the brain parenchyma but in the subarachnoid space, extending to epidural space. It is possible that schwannomas could arise from small nerves in the subarachnoid space or the leptomeninges. Although several cases of schwannomas attached to the dura have been reported[
The imaging findings in our case were similar to convexity meningioma; however, there were no evident findings of “dural tail sign,” but the enhanced lesion extending along the meningeal or subarachnoid space; this location for schwannomas is exceedingly rare.
The differential diagnosis includes subdural, epidural, or leptomeningeal nodular brain metastasis, meningioma, glioma, and some granulomatous lesions (such as tuberculoma, fungal infections, and parasitic infections) when a well-demarcated spherical lesion associated with ring enhancement is observed adjacent to the cerebral convexity.
These lesions are uncommon, except for meningiomas, gliomas, and metastases.
CONCLUSION
We have reported on a middle-age man with a schwannoma located in the subarachnoid space and have presented computed tomography and MRI findings. Although there was no diagnostic clue indicating a convexity schwannoma (because of the rarity and nonspecific imaging features of such tumors), it should be recognized that schwannomas may arise in such rare locations as the subarachnoid space.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. AlbBatly AA, ZAkzouk RS, Alhaidey AK. An atypical case of intracerebral schwannoma. Pan Afr Med J. 2014. 18: 342-
2. Aryanpur J, Long DM. Schwannoma of the medulla oblongata. Case report. J Neurosurg. 1988. 69: 446-9
3. Bardosa MD, Rebelo O, Barbosa P, Goncalves J, Fernandes R. Cystic intraventricular schwannoma: Case report and review of the literature. Neurocirugia (Astur). 2001. 12: 56-60
4. Benedict WJ, Brown HG, Sivarajan G, Prabhu VC. Intraventricular schwannoma in a 15-year-old adolescent: A case report. Childs Nerv Syst. 2008. 24: 529-32
5. Casadei GP, Komori T, Scheithauer BW, Miller GM, Parisi JE, Kelly PJ. Intracranial parenchymal schwannoma. A clinicopathological and neuroimaging study of nine cases. J Neurosurg. 1993. 79: 217-22
6. Celikoglu E, Hakan T, Bozbuga M. Cystic schwannoma of the falx cerebri. J Clin Neurosci. 2007. 14: 589-92
7. Consales A, Rossi A, Nozza P, Raveqnani M, Garre ML, Cama A. Intracerebral schwannoma in a child. Br J Neurosurg. 2010. 24: 306-8
8. Di Biasi C, Trasimeni G, Iannilli M, Polettini E, Gualdi GF. Intracerebral schwannoma: CT and MR findings. AJNR Am J Neuroradiol. 1994. 15: 1956-8
9. Dow GR, Hussein A, Robertson IJ. Supratentorial intraventricular schwannoma. Br J Neurosurg. 2004. 18: 561-2
10. Erdogan E, Onguru O, Bulakbasi N, Baysefer A, Gezen F, Timurkaynak E. Schwannoma of the lateral ventricle: Eight-year foolow-up and literature review. Minim Invasive Neurosurg. 2003. 46: 50-3
11. Erongun U, Ozkal E, Acar O, Uygun A, Kocaoqullar Y, Gungor S. Intracerebral schwannoma: Case report and review. Neurosurg Rev. 1996. 19: 269-74
12. Ezura M, Ikeda H, Ogawa A, Yoshimoto T. Intracerebral schwannoma: Case report. Neurosurgery. 1992. 30: 97-100
13. Feigin I, Ogata J. Schwann cells and peripheral myelin within human central nervous tissues: The mesenchymal character of Schwann cells. J Neuropathol Exp Neurol. 1971. 30: 603-12
14. Frim DM, Ogilvy CS, Vonsattal JP, Chapman PH. Is intracerebral schwannoma a developmental tumor of children and young adults? Case report and review. Pediatr Neurosurg. 1992. 18: 190-4
15. Gao Y, Qin Z, Li D, Yu W, Sun L, Liu N. Intracerebral schwannoma: A case report and literature review. Oncol Lett. 2018. 16: 2501-10
16. Ghatak NR, Norwood CW, Davis CH. Intracerebral schwannoma. Surg Neurol. 1975. 3: 45-7
17. Ghosh S, Chandy MJ. Solitary ectopic intracerebral schwannoma. Br J Neurosurg. 1992. 6: 163-6
18. Gibson AA, Hendrick EB, Copen PE. Case reports. Intracerebral schwannoma. Report of a case. J Neurosurg. 1966. 24: 552-7
19. Gokay H, Izgi N, Barlas O, Erseven G. Supratentorial intracerebral schwannomas. Surg Neurol. 1984. 22: 69-72
20. Guha D, Kiehl TR, Krings T, Valiante TA. Intracerebral schwannoma presenting as classic temporal epilepsy. J Neurosurg. 2012. 117: 136-40
21. Ishihara M, Miyagawa-Hayashino A, Nakashima Y, Haga H, Takahashi JA, Manabe T. Intracerebral schwannoma in a child with infiltration along perivascular spaces resembling meningioangiomatosis. Pathol Int. 2009. 59: 583-7
22. Jaimovich R, Jaimovich SG, Arakaki N, Sevlever G. Supratentorial intraventricular solitary schwannoma. Case report and literature review. Childs Nerv Syst. 2013. 29: 499-504
23. Jung JM, Shin HJ, Chi JG, park IS, Kim ES, Han JW. Malignant intraventricular schwannoma. Case report. J Neurosurg. 1995. 82: 121-4
24. Kasantikul V, Brown WJ, Cahan LD. Intracerebral neurilemmoma. J Neurol Neurosurg Psychiatry. 1981. 44: 1110-5
25. Landouceur D, Bergeron D, Lamarche JB, Lamontagne L. Cystic schwannoma of the brainstem. Can J Neurol Sci. 1989. 16: 357-60
26. Lee S, Park SH, Chung CK. Supratentorial intracerebral schwannoma: Its fate and proper management. J Korean Neurosurg Soc. 2013. 54: 340-3
27. Lin J, Feng H, Li F, Zhao B, Guo Q. Intraparenchymal schwannoma of the medulla oblongata. Case report. J Neurosurg. 2003. 98: 621-4
28. Ma L, Yang SX, Wang YR. Intracerebral schwannoma mimicking parasagittal meningioma. J Craniofac Surg. 2013. 24: e541-3
29. Maiuri F, Colella G, D’Acunzi G, De Caro Mdel B. Malignant intracerebellar schwannoma. J Neurooncol. 2004. 66: 191-5
30. Messing-Junger AM, Riemenschneider MJ, Reifenberger G. A 21-year-old female with a third ventricular tumor. Brain Pathol. 2006. 16: 87-8
31. Muzzafar S, Ketonen L, Weinberg JS, Schllingerhout D. Imaging and clinical features of an intra-axial brain stem schwannoma. AJNR Am J Neuroradiol. 2010. 31: 567-9
32. Nakayama K, Nakayama T, Matsuoka Y, Kono K. Supratentorial convexity leptomeningeal schwannoma: Case report. Neurosurgery. 2002. 51: 1295-8
33. Nelson E, Rennels M. Innervation of intracranial arteries. Brain. 1970. 93: 475-90
34. New PF. Intracerebral schwannoma. Case report. J Neurosurg. 1972. 36: 795-7
35. Oertel MF, Nolte KW, Blaum M, Weis J, Gilsbach JM, Korinth MC. Primary intraventricular schwannomas. Clin Neurol Neurosurg. 2009. 111: 768-73
36. Ost AH, Meyer R. Cystic intraventricular schwannoma: A case report. AJNR Am J Neuroradiol. 1990. 11: 1262-4
37. Paredes I, Roldan LJ, Ramos A, Lobato RD, Ricoy JR. Intraparenchymal schwannomas: Report of two new cases studied with MRI and review of the literature. Clin Neurol Neurosurg. 2012. 114: 42-6
38. Pimentel J, Tavora L, Cristina ML, Antunes JA. Intraventricular schwannoma. Childs Nerv Syst. 1998. 4: 373-5
39. Prakash B, Roy S, Tandon PN. Schwannoma of the brain stem: Case report. J Neurosurg. 1980. 53: 121-3
40. Raswan US, Bhat I, Samoon N, Arif SH, Laharwal M, Chhiber SS. Supratentorial extraparenchymal schwannoma mimicking parasagittal meningioma: A rare case report. Surg Neurol Int. 2017. 8: 228-
41. Redekop G, Elisevich K, Glibert J. Fourth ventricular schwannoma. Case report. J Neurosurg. 1990. 73: 777-81
42. Riggs HE, Clary WU. A case of intramedullary sheath cell tumor of the spinal cord: Consideration of vascular nerves as a source of origin. J Neuropathol Exp Neurol. 1957. 16: 332-6
43. Rodriguez-Salazar A, Carrillo R, de Miguel J. Intracerebral schwannoma in a child: Report of a case. Childs Brain. 1984. 11: 69-72
44. Russell DS, Rubinstein DS, Russell DS, Rubinstein LJ.editors. Schwannomas. Russell and Rubinstein’s Pathology of Tumors of the Nervous System. London: Arnold Publisher; 1989. 5: 535-60
45. Sarkar C, Mehta VS, Roy S. Intracerebellar schwannoma. Case report. J Neurosurg. 1987. 67: 120-3
46. Schwartz AM, Sotrel A. Intracerebral and intracerebellar neurilemoma. South Med J. 1988. 81: 385-8
47. Shalit MN, Toledo E, Sandbank U. Intracerebral schwannoma. Acta Neurochir (Wien). 1982. 64: 253-8
48. Sharma MC, Karak AK, Gaikwad SB, Mahapatra AK, Mehta VS, Sudha K. Intracranial intraparenchymal schwannomas: A series of eight cases. J Neurol Neurosurg Psychiatry. 1996. 60: 200-3
49. Sharma RR, Gurusinqhe NT, Lynch PG, Parekh HC, Bertolis G. Intraparenchymatous schwannoma of the cerebellum. Br J Neurosurg. 1993. 7: 83-9
50. Sharma V, Newton G. Schwannoma of the medulla oblongata. Br J Neurosurg. 1993. 7: 427-9
51. Srinivas R, Krupashnkar D, Shasi V. Intracerebral schwannoma in a 16-year-old girl: A case report and review of the literature. Case Rep Neurol Med. 2013. 2013: 171494-
52. Stefanko SZ, Vuzevski VD, Maas AI, van Vroonhoven CC. Intracerebral malignant schwannoma. Acta Neuropathol. 1986. 71: 321-5
53. Tanabe M, Miyata H, Okamoto H, Watanabe T, Hori T, Masakawa A. Brainstem schwannoma-case report. Neurol Med Chir (Tokyo). 1996. 36: 880-3
54. Ten H, Adachi K, Yamaguchi F, Matsuno A, Teramoto A, Morita A. Occipital lobe epilepsy was presented in a patient with intracerebral schwannoma: A case report and literature review. Int J Neuosci. 2019. 129: 308-12
55. Tran-Dinh HD, Soo YS, O’Neil P, Chaseling R. Cystic cerebellar schwannoma: Case report. Neurosurgery. 1991. 29: 269-9
56. Tsuiki H, Kuratsu J, Ishimaru Y, Nakahara T, Kishida K, Takamura M. Intracranial intraparenchymal schwannoma: Report of three cases. Acta Neurochir (Wien). 1997. 139: 756-60
57. Van Rensburg MJ, Proctor NS, Danziger J, Orelowitz MS. Temporal lobe epilepsy due to an intracerebral schwannoma: Case report. J Neurol Neurosurg Psychiatry. 1975. 38: 703-9
58. Weiner HL, Zaqzaq D, Babu R, Weinreb HJ, Ransohoff J. Schwannoma of the fourth ventricle presenting with hemifacial spasm. A report of two cases. J Neurooncol. 1993. 15: 37-43
59. Wilson BR, Steinberg JA, Snyder V, Jiang MN, Carter BS. Histologic evidence for arteriovenous malformation-like vasculature occurring within an intracerebral schwannoma: A case report and review of the literature. World Neurosurg. 2016. 92: 582.e9-13
60. Zagardo MT, Castellani RJ, Rees JH, Rothman MI, Zoarski GH. Radiologic and pathologic findings of intracerebral schwannoma. AJNR Am J Neuroradiol. 1998. 19: 1290-3