- Department of Neurosurgery, University of Miami, Miami, Florida, United States
- Departments of Pathology, Santa Clara Valley Medical Center, San Jose, California, United States.
- Departments of Radiology, Santa Clara Valley Medical Center, San Jose, California, United States.
- Departments of Neurosurgery, Santa Clara Valley Medical Center, San Jose, California, United States.
Correspondence Address:
Harminder Singh
Departments of Neurosurgery, Santa Clara Valley Medical Center, San Jose, California, United States.
DOI:10.25259/SNI_188_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anil K. Mahavadi, Caroline Temmins, Mahesh R. Patel, Harminder Singh. Supratentorial intraventricular rosette-forming glioneuronal tumors – Case report and review of treatment paradigms. 06-Jun-2020;11:138
How to cite this URL: Anil K. Mahavadi, Caroline Temmins, Mahesh R. Patel, Harminder Singh. Supratentorial intraventricular rosette-forming glioneuronal tumors – Case report and review of treatment paradigms. 06-Jun-2020;11:138. Available from: https://surgicalneurologyint.com/surgicalint-articles/10066/
Abstract
Background: Rosette-forming glioneuronal tumors (RGNT) are slow-growing WHO Grade I tumors that are characterized by mixed histology and rosette formation. Although typically located in the posterior fossa, these tumors can rarely originate elsewhere. Here, we describe the fourth case in literature where an RGNT was localized to the lateral ventricles and detail the treatment approach.
Case Description: A 41-year-old male presented with a 10 day history of gradually worsening headaches and mild gait difficulty. Computed tomography and magnetic resonance imaging (MRI) identified a heterogeneously enhancing 6.0 cm left lateral ventricular cystic mass with hydrocephalus. An interhemispheric transcallosal approach was performed for tumor debulking. The mass was emanating from the roof of the left lateral ventricle. Sub-total resection (STR) was achieved. Pathology showed a glioneuronal neoplasm with vague neurocytic rosettes and loose perivascular pseudorosettes. Tumor vessels were thickly hyalinized and contained eosinophilic granular bodies and Rosenthal fibers. Tumor stained positive for GFAP, S-100, OLIG2, and SOX10, and patchy positive for epithelial membrane antigen (EMA), D2-40, CD99, and p16. Neurocytic rosettes and perivascular structures stained positive for synaptophysin. The patient was discharged home uneventfully and remained intact at his 6-month follow-up visit. Long-term care included MRI surveillance with repeat surgery being considered in case of progression.
Conclusion: In this report, we describe the fourth case of an RGNT being isolated to the lateral ventricles and the first where it stained positive for EMA and D2-40. Our patient’s uneventful recovery after STR indicates that surgery alone continues to be a viable initial treatment option.
Keywords: Lateral ventricle, Outcomes, Rosette-forming glioneuronal tumor, Sub-total resection, Supratentorial
INTRODUCTION
Rosette-forming glioneuronal tumors (RGNT) are rare, slow growing mixed cell tumors. RGNTs were first described as a distinct entity in 2002 and added to the WHO classification of tumors of the central nervous system in 2007 as a Grade I tumor alongside the papillary glioneuronal tumor.[
RGNTs typically occur in young adults between the ages of 25 and 33 and display a preference for the posterior fossa. They can lead to gradually worsening symptoms of headaches, ataxia, and vertigo, from both local mass effect and cerebrospinal fluid (CSF) obstruction.[
A meta-analysis of cases compiled until December 2012 found that out of 85 RGNTs, 80% (68) of them occurred in the posterior fossa while only 15.3% (13) occurred supratentorially.[
Here, we present the unusual case of a histopathologically confirmed RGNT which originated from the lateral ventricle but which also demonstrated infiltrative properties. To the best of our knowledge, this is only the fourth such case in the literature. The unique presentation of this patient and his subsequent hospital course may be useful in guiding future discussions around the management of this rare tumor.
CASE REPORT
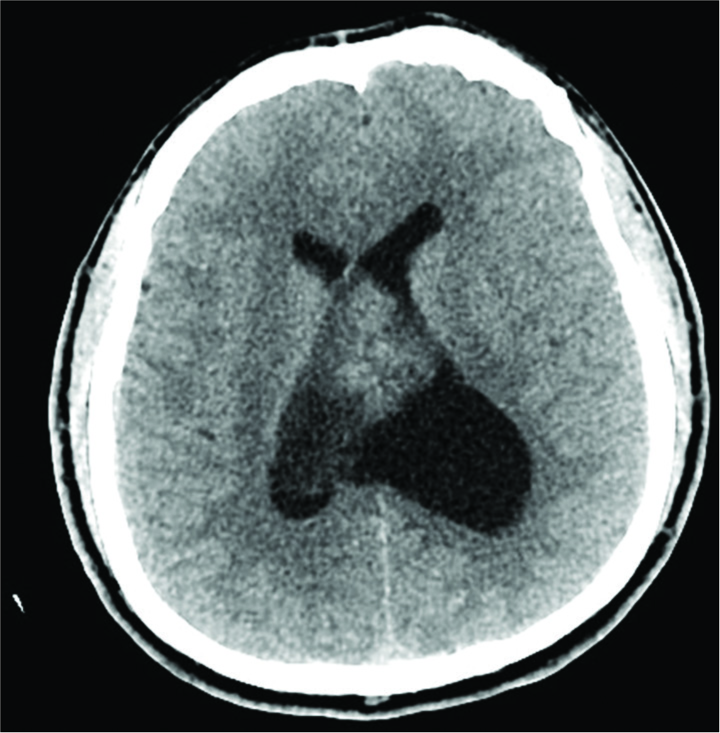
A 41-year-old male with no significant medical history presented to an outside hospital with a 10 day history of continuous headaches, initially rated as 2/10 but occasionally rising to 4/10 on the visual analog pain scale. His headaches were localized to the right occipital region and were unresponsive to acetaminophen and ibuprofen. He had some mild gait difficulty but no nausea or emesis. A computed tomography (CT) scan identified a mixed density 6.0 cm intraventricular mass with dilatation of the left lateral ventricle and a 1.4 cm rightward bowing of the intraventricular septum [
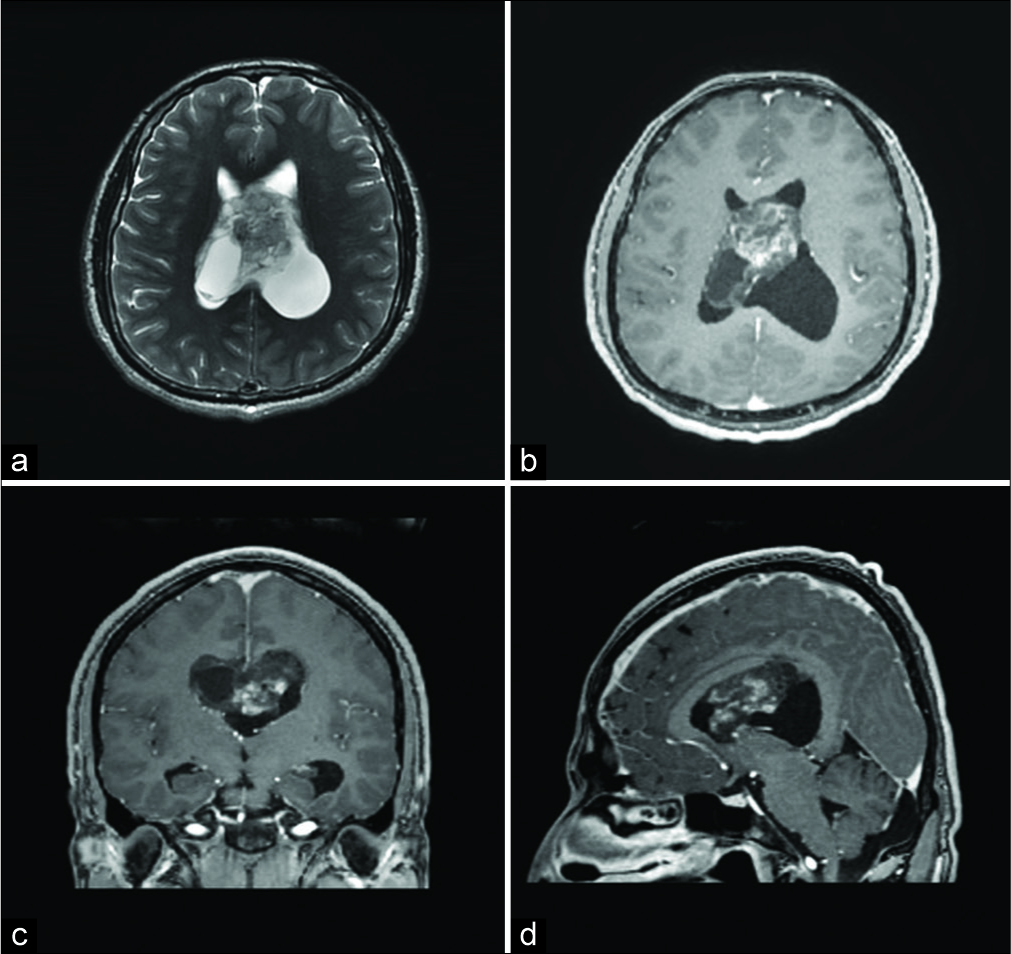
He was started on dexamethasone and referred to our hospital for further workup. He underwent a preoperative magnetic resonance imaging (MRI) of the brain, with and without gadolinium, which showed a heterogeneously enhancing lesion with a cystic component. The lesion was emanating from the roof of the left lateral ventricle from the region of the corpus callosum [
Figure 2:
(a) Axial T2-weighted magnetic resonance imaging (MRI) shows a heterogeneous intraventricular lesion containing T2-hypointense components anteriorly and a cystic component at the right posterior aspect. Postcontrast axial (b), coronal (c) and sagittal (d) T1-weighted MRI sequences show a heterogeneously enhancing mass following intravenous contrast. The sagittal image also shows extension of the posterosuperior extent of the lesion in the undersurface of the posterior body of the corpus callosum.
On neurological examination, there were no significant deficits.
It was decided to surgically resect the lesion. The primary goal of the surgery was to debulk the majority of the lesion and restore CSF flow pathways. Therefore, we chose to approach the lesion along its long axis through the left frontal horn of the ventricle. The patient was counseled preoperatively that a second staged parieto-occipital approach might be necessary to remove the remainder of the lesion, depending on the pathology and the extent of resection during the initial surgery. A bifrontal craniotomy with the left interhemispheric transcallosal approach was performed for resection of the tumor. The interhemispheric approach was chosen over the transcortical trans-ventricular approach because of the extension of the tumor into bilateral lateral ventricles. The interhemispheric approach, being a midline approach, gave us access to bilateral ventricles without significant brain retraction and without traversing the cerebral cortex. An endoscopic portal approach was not used considering the size and extension of the tumor into the lateral ventricles. While the endoscopic approach might have been well suited for a biopsy of the lesion, it is not optimal for debulking and dissection of large lesions.
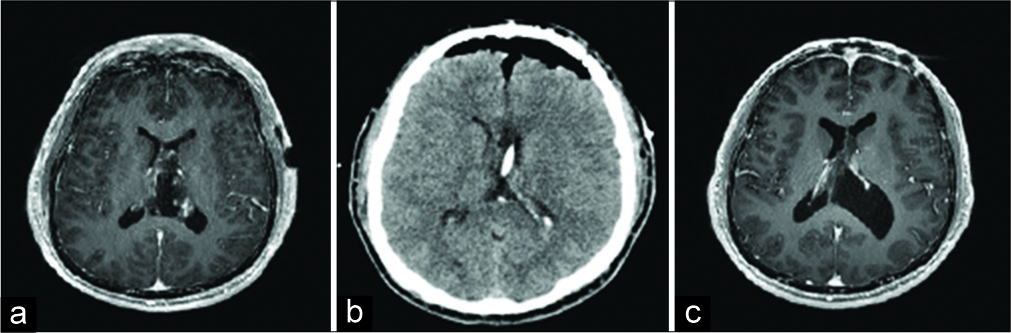
Intraoperatively, using a left interhemispheric approach, a small (<1.5 cm) corpus callosotomy was performed to enter the left frontal horn of the lateral ventricle where the tumor was debulked using bipolar cautery, suction, as well as a cavitron ultrasonic aspirator (CUSA). Within the left lateral ventricle, there was large cyst that was exerting mass effect on the septum pellucidum and displacing it into the right lateral ventricle. The cyst was drained. The solid portion of the tumor was emanating from the lateral wall and roof of the left ventricle, from the region of the posterior corpus callosum. The CUSA was used debulk the solid portion of the tumor. On debulking of the tumor, the ventricle walls collapsed inward and further resection was carried out cautiously because of tumor invasion into the ependymal walls of the left lateral ventricle. The deep venous drainage of the brain was embedded within the caudal aspect of the tumor extending into the third ventricle. These vessels were carefully preserved. On conclusion of the resection, an external ventricular drain (EVD) was left behind in the left lateral ventricle. The EVD was weaned and removed a couple of days after surgery. Immediate postoperative noncontrast CT and T1 with contrast MRI showed sub-total resection (STR) of tumor with resolution of mass effect and no postoperative hydrocephalus [
Figure 3:
Postoperative imaging. (a) Postcontrast axial T1-weighted image shows resolution of hydrocephalus with a small residual intraventricular enhancing mass consistent with sub-total resection. (b) Axial noncontrast head computed tomography scan shows postoperative pneumocephalus with residual tumor better seen on magnetic resonance imaging. A partially visualized shunt traversing the body of the left lateral ventricle is seen with resolution of previous hydrocephalus. (c) 6-month postcontrast axial T1-weighted image shows stable lesion with minimal enhancement.
The patient was ultimately discharged home within a week and toward the end of his stay, was ambulating freely without any complaints and possessed an intact neurological exam as assessed by the discharge physician. He was seen in clinic at 3- and 6-months follow-up postsurgery. He had complete resolution of his headaches and remained neurologically intact without any deficits. His 6-month postoperative MRI showed stable to slightly decreased size of residual tumor with minimal enhancement, markedly decreased from prior [
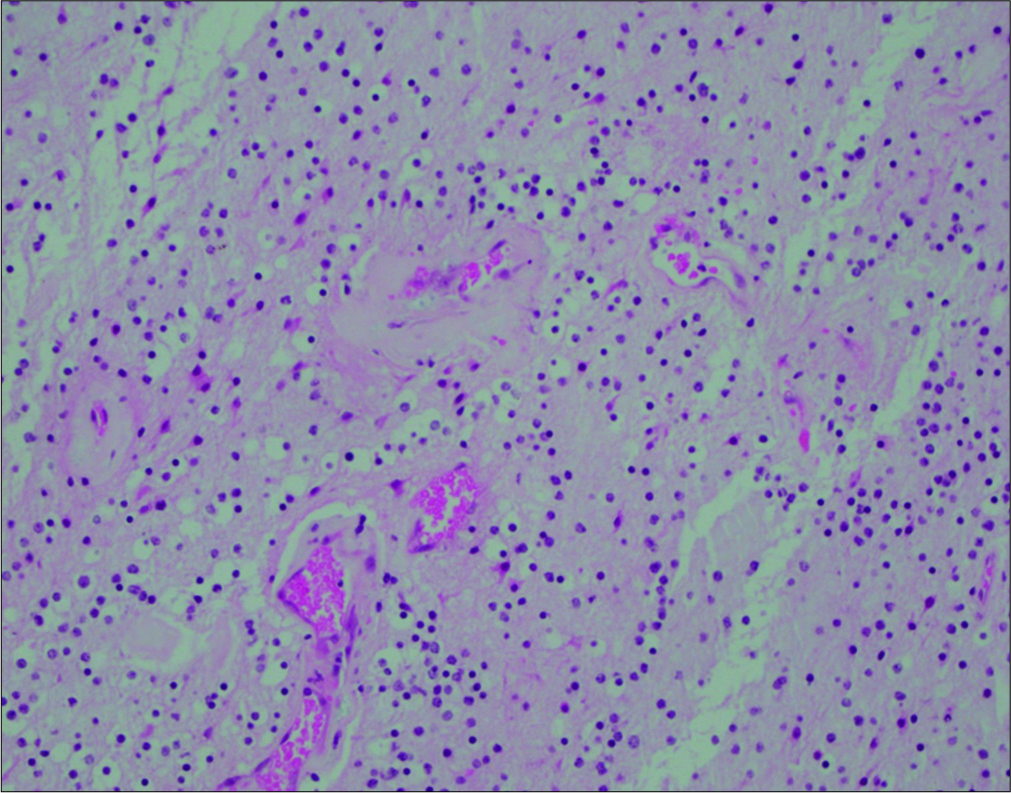
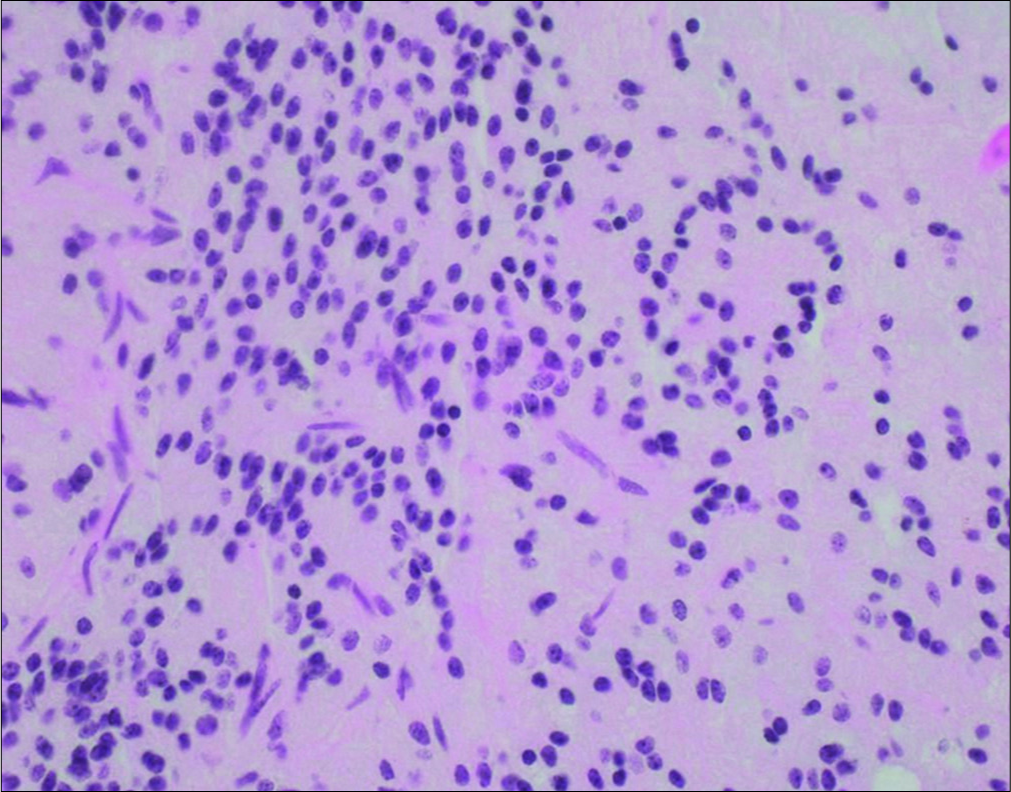
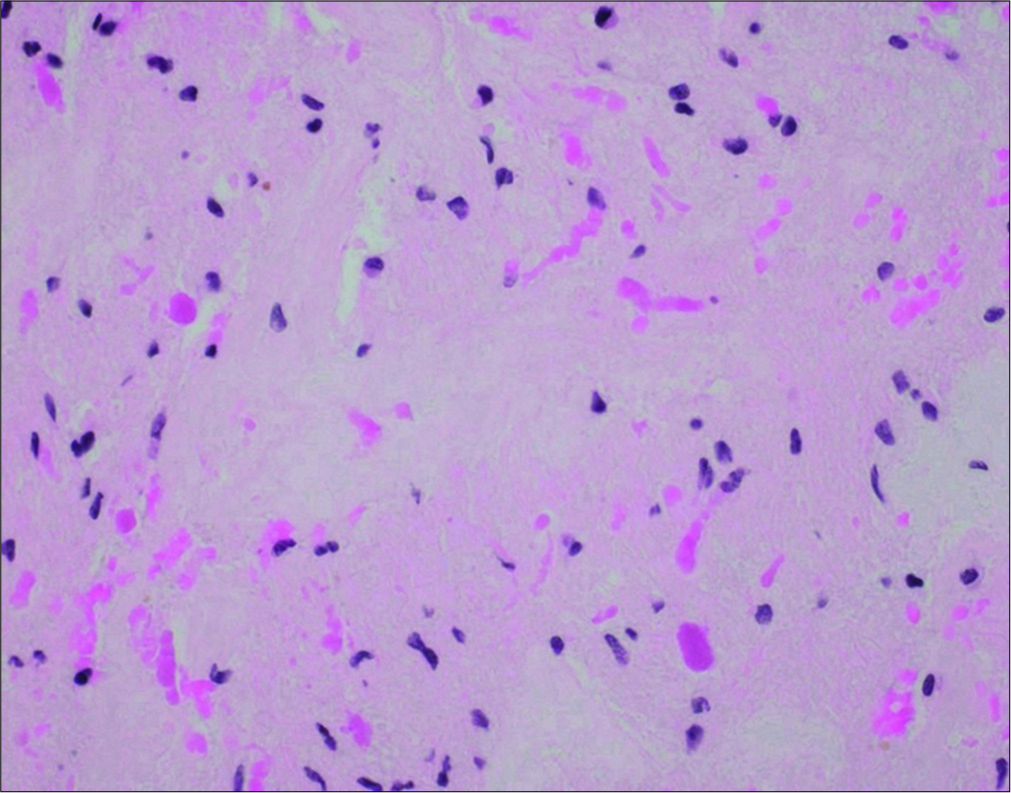
Pathology showed a solid-appearing, glioneuronal neoplasm composed of monotonous cells with round nuclei, punctate chromatin, and cytoplasmic clearing in a neuropil-like background. The tumor cells were arranged in vague neurocytic rosettes and loose perivascular pseudorosettes in a lightly myxoid background. Vessels within the tumor were seen to be thickly hyalinized and there were focal piloid areas with eosinophilic granular bodies and Rosenthal fibers [
DISCUSSION
In this report, we detail the disease course and treatment of a 41-year-old patient who presented with an infiltrative RGNT of the left lateral ventricle. To the best of our knowledge, this is only the fourth case in literature, where the tumor was restricted to the lateral ventricles.
As the name implies, RGNTs are characterized by the presence of glial and neurocytic cells with a neuronal rosette and/or perivascular pseudorosette component and a glial component.[
Clinical presentation
RGNTs predominantly affect young adults with a mean age range of 24.8–33 years, although cases as young as 12 and as old as 70 have been reported.[
Occasionally, the patients can present acutely due to complications associated with tumor growth, as in a 20-year-old patient who developed emergent symptoms of obstructive hydrocephalus secondary to intratumoral hemorrhage after the RGNT likely necrosed on outgrowing its vascular supply.[
Treatment and follow-up
Similar to other symptomatic low grade gliomas, RGNTs can be suitable candidates for surgical resection and the vast majority of patients (>90%) undergo either gross- total resection (GTR) or STR resection, and can remain progression-free for up to 30.2 months.[
Yang et al.’s 2017 review of 141 RGNT cases found that GTR was performed in 48.9%, incomplete resection (STR and partial) in 37.5%, while biopsy was performed in 13.5% of patients. Only 1.4% of patients underwent chemotherapy, and 4.3% underwent radiotherapy (RT). STR progression- free survival (PFS) was found to be comparable to GTR (hazard ratio [HR] 0.655, 95% confidence interval [CI] 0.028–15.569, P = 0.793), while the mixed group of biopsy and partial resection was found to be associated with higher rates of tumor progression compared to GTR (HR 98.258, 95% CI 1.339–7211.531, P = 0.036 and HR 155.496, 95% CI 4.336–5575.730, P = 0.006, respectively). They also showed that progression was less likely in adult patients than in pediatric patients (HR 0.003, 95% CI 0.000–0.181, P = 0.005) and that the risk of progression was higher in solid RGNTs that in ones with cystic components (HR 78.739, 95% CI 1.479–4192.776, P = 0.031).[
Interestingly, even though these tumors are assigned as WHO Grade I, infiltration of surrounding structures and ventricular infiltration is frequently observed and can lead to rather high complication rates (>45%) on resection.[
Schlamann et al. performed an earlier literature review in 2014 which cites three STR patients receiving a median of 55 Gy through focal RT but did not provide their specific PFS and overall survival (OS). For the 52 patients in this particular paper of a pooled cohort with outcome data, PFS and OS rates at 2 years were both 100%. The favorable prognosis of RGNTs is also reflected by the low overall rates of progression and death at long-term follow-up, which was 11.6% and 4.7%, respectively, with a mean follow-up of 28.5 months.[
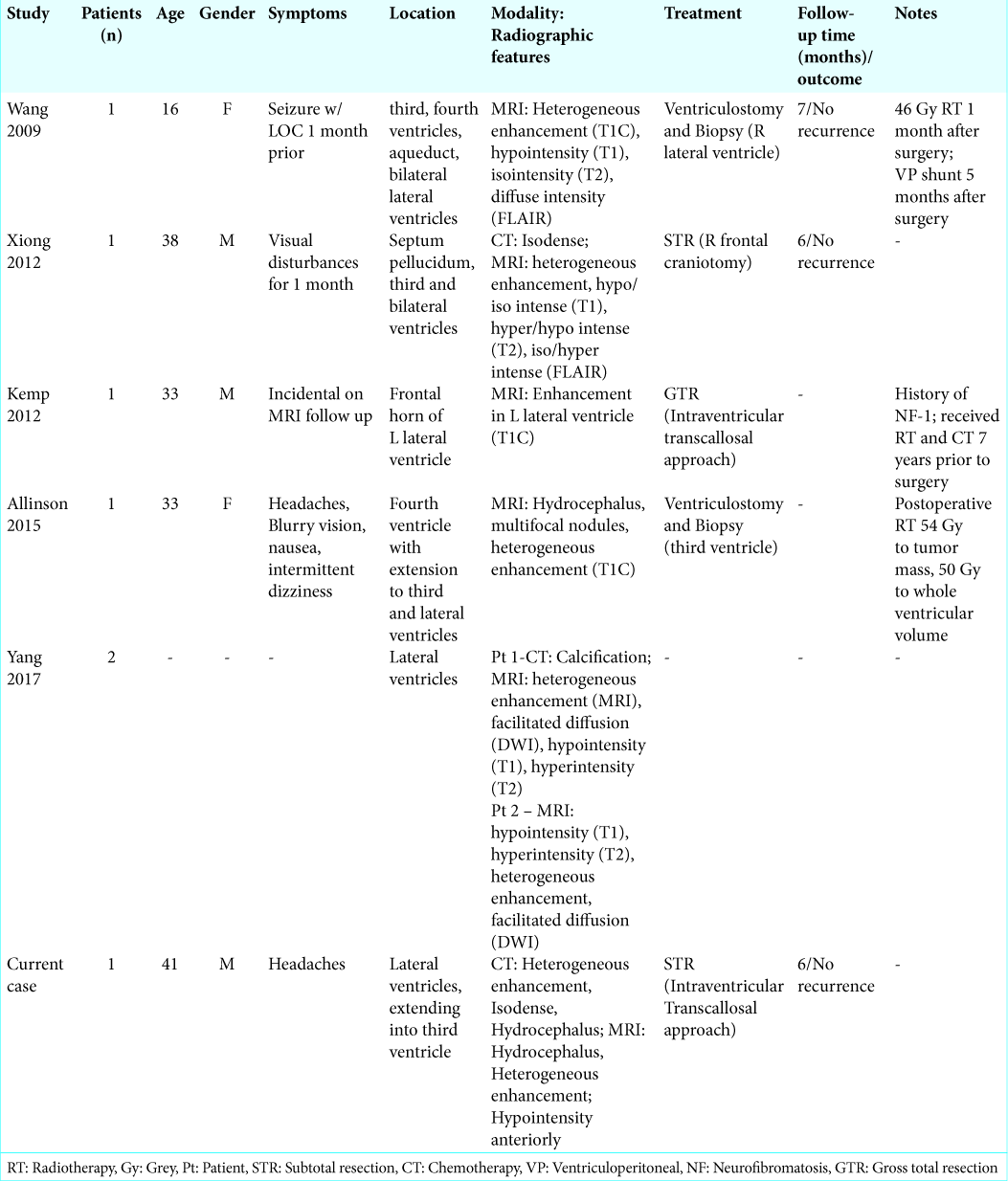
Within the five cases of the lateral ventricle cohort with reported data, only 60% (3/5) underwent surgery for resection [
Pathology and molecular profile
The characteristic histological profile of neurocytic rosettes and perivascular pseudorosettes in a glial setting was observed in our patient. This was confirmed by the positive stains for GFAP, S-100, OLIG2, and SOX10 in the glial component, with synaptophysin staining neurocytic rosettes and perivascular structures. Interestingly, while EMA was found to be absent in a large systematic review of 57 RGNT cases, EMA was weakly positive in our patient.[
CONCLUSION
Given the uneventful and sustained neurological recovery of our patient, we demonstrate that STR continues to be a safe and effective treatment option for RGNTs. Further research into the risk factors that predispose these tumors to originate outside of the posterior fossa in uncharacteristic locations may be warranted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Allende DS, Prayson RA. The expanding family of glioneuronal tumors. Adv Anat Pathol. 2009. 16: 33-9
2. Allinson KS, O’Donovan DG, Jena R, Cross JJ, Santarius TS. Rosette-forming glioneuronal tumor with dissemination throughout the ventricular system: A case report. Clin Neuropathol. 2015. 34: 64-9
3. Bera G, Das A, Chatterjee S, Chatterjee U. Rosette-forming glioneuronal tumor: A rare posterior fossa tumor in an adolescent. J Pediatr Neurosci. 2017. 12: 168-71
4. Bidinotto LT, Scapulatempo-Neto C, Mackay A, de Almeida GC, Scheithauer BW, Berardinelli GN. Molecular profiling of a rare rosette-forming glioneuronal tumor arising in the spinal cord. PLoS One. 2015. 10: e0137690-
5. Cabezas SG, Blanch RS, Sanchez-Sanchez R, Eito AJ. Rosette-forming glioneuronal tumour (RGNT) of the fourth ventricle: A highly aggressive case. Brain Tumor Pathol. 2015. 32: 124-30
6. Hasselblatt M, Paulus W. Sensitivity and specificity of epithelial membrane antigen staining patterns in ependymomas. Acta Neuropathol. 2003. 106: 385-8
7. Kemp S, Achan A, Ng T, Dexter MA. Rosette-forming glioneuronal tumour of the lateral ventricle in a patient with neurofibromatosis 1. J Clin Neurosci. 2012. 19: 1180-1
8. Komori T, Scheithauer BW, Hirose T. A rosette-forming glioneuronal tumor of the fourth ventricle: Infratentorial form of dysembryoplastic neuroepithelial tumor?. Am J Surg Pathol. 2002. 26: 582-91
9. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
10. Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993. 11: 173-89
11. Marhold F, Preusser M, Dietrich W, Prayer D, Czech T. Clinicoradiological features of rosette-forming glioneuronal tumor (RGNT) of the fourth ventricle: Report of four cases and literature review. J Neurooncol. 2008. 90: 301-8
12. Morris C, Prudowsky ZD, Shetty V, Geller T, Elbabaa SK, Guzman M. Rosette-forming glioneuronal tumor of the fourth ventricle in children: Case report and literature review. World Neurosurg. 2017. 107: 1045.e9-1045.e1016
13. Nakamura Y, Kanemura Y, Yamada T, Sugita Y, Higaki K, Yamamoto M. D2-40 antibody immunoreactivity in developing human brain, brain tumors and cultured neural cells. Mod Pathol. 2006. 19: 974-85
14. Podlesek D, Geiger K, Hendry DJ, Schackert G, Krex D. Rosette-forming glioneuronal tumor of the fourth ventricle in an elderly patient. J Neurooncol. 2011. 103: 727-31
15. Scheithauer BW, Silva AI, Ketterling RP, Pula JH, Lininger JF, Krinock MJ. Rosette-forming glioneuronal tumor: Report of a chiasmal-optic nerve example in neurofibromatosis Type 1: Special pathology report. Neurosurgery. 2009. 64: E771-2
16. Schlamann A, von Bueren AO, Hagel C, Zwiener I, Seidel C, Kortmann RD. An individual patient data meta-analysis on characteristics and outcome of patients with papillary glioneuronal tumor, rosette glioneuronal tumor with neuropil-like islands and rosette forming glioneuronal tumor of the fourth ventricle. PLoS One. 2014. 9: e101211-
17. Sequerra EB. Subventricular zone progenitors in time and space: Generating neuronal diversity. Front Cell Neurosci. 2014. 8: 434-
18. Shah MN, Leonard JR, Perry A. Rosette-forming glioneuronal tumors of the posterior fossa. J Neurosurg Pediatr. 2010. 5: 98-103
19. Sieg EP, Payne R, Langan S, Specht CS. Case report: A rosette-forming glioneuronal tumor in the tectal plate in a patient with neurofibromatosis Type I. Cureus. 2016. 8: e857-
20. Slowik F, Pásztor E, Szöllösi BJ. Subependymal gliomas. Neurosurg Rev. 1979. 2: 79-86
21. Smith AB, Smirniotopoulos JG, Horkanyne-Szakaly I. From the radiologic pathology archives: Intraventricular neoplasms: Radiologic-pathologic correlation. Radiographics. 2013. 33: 21-43
22. Solis OE, Mehta RI, Lai A, Mehta RI, Farchoukh LO, Green RM. Rosette-forming glioneuronal tumor: A pineal region case with IDH1 and IDH2 mutation analyses and literature review of 43 cases. J Neurooncol. 2011. 102: 477-84
23. Tan CC, Gonzales M, Veitch A. Clinical implications of the infratentorial rosette-forming glioneuronal tumor: Case report. Neurosurgery. 2008. 63: E175-6
24. Vajtai I, Arnold M, Kappeler A, Jeless O, Lukes A, Mariani L. Rosette-forming glioneuronal tumor of the fourth ventricle: Report of two cases with a differential diagnostic overview. Pathol Res Pract. 2007. 203: 613-9
25. van den Bent MJ, Snijders TJ, Bromberg JE. Current treatment of low grade gliomas. Memo. 2012. 5: 223-7
26. Wang Y, Xiong J, Chu SG, Liu Y, Cheng HX, Wang YF. Rosette-forming glioneuronal tumor: Report of an unusual case with intraventricular dissemination. Acta Neuropathol. 2009. 118: 813-9
27. Xiong J, Liu Y, Chu SG, Chen H, Chen HX, Mao Y. Rosette-forming glioneuronal tumor of the septum pellucidum with extension to the supratentorial ventricles: Rare case with genetic analysis. Neuropathology. 2012. 32: 301-5
28. Xu J, Yang Y, Liu Y, Wei M, Ren J, Chang Y. Rosette-forming glioneuronal tumor in the pineal gland and the third ventricle: A case with radiological and clinical implications. Quant Imaging Med Surg. 2012. 2: 227-31
29. Yang C, Fang J, Li G, Li S, Ha T, Wang J. Histopathological, molecular, clinical and radiological characterization of rosette-forming glioneuronal tumor in the central nervous system. Oncotarget. 2017. 8: 109175-90
30. Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013. 45: 602-12